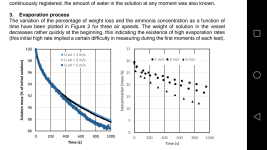
Jagube
Esteemed member
When you put an acid in water, it dissociates, giving off H+ ions (HA -> H+ + A-).
Similarly, when you put a base in water, it dissociates, giving off OH- ions (B + H2O -> BH+ + OH−).
It's the H+ and OH- ions that make the solution acidic or basic. It's these ions we want. The dissociated molecule (A- or BH+) is garbage.
In the case of some acids and bases, the garbage molecule evaporates. Some common examples are acetic acid and ammonia. As you evaporate solutions of those, they smell, but eventually the smell disappears.
But what about the ions (H+ or OH-)? My understanding is that they don't evaporate. That means you can't neutralize your soup by evaporating it.
In particular:
- If you cook Ayahuasca with a lot of vinegar, and reduce your tea, it will only become more acidic, because the volume will decrease, but the amount of H+ ions will remain the same. The evaporation will rid it of its vinegar smell and taste, but not its acidity. And because of the smaller volume, it will become madly acidic.
- If you use ammonia to base your harmala alkaloids and evaporate it, the remaining powder will not have any ammonia in it, but it will be full of OH- ions and therefore be very alkaline... probably too alkaline to ingest safely without neutralizing first.
Is my understanding correct?
Similarly, when you put a base in water, it dissociates, giving off OH- ions (B + H2O -> BH+ + OH−).
It's the H+ and OH- ions that make the solution acidic or basic. It's these ions we want. The dissociated molecule (A- or BH+) is garbage.
In the case of some acids and bases, the garbage molecule evaporates. Some common examples are acetic acid and ammonia. As you evaporate solutions of those, they smell, but eventually the smell disappears.
But what about the ions (H+ or OH-)? My understanding is that they don't evaporate. That means you can't neutralize your soup by evaporating it.
In particular:
- If you cook Ayahuasca with a lot of vinegar, and reduce your tea, it will only become more acidic, because the volume will decrease, but the amount of H+ ions will remain the same. The evaporation will rid it of its vinegar smell and taste, but not its acidity. And because of the smaller volume, it will become madly acidic.
- If you use ammonia to base your harmala alkaloids and evaporate it, the remaining powder will not have any ammonia in it, but it will be full of OH- ions and therefore be very alkaline... probably too alkaline to ingest safely without neutralizing first.
Is my understanding correct?