..i initially began pondering the mysterious Mimosa hostilis alkaloid yuremamine
in What are the current views, facts and theories on DMT-N-OXIDE? - DMT Discussion - Welcome to the DMT-Nexus(see posts #11 and #13 for detail and refs) .. endlessness noted that it contains within it a DMT molecule..it is broken down into constituents by heat and basic conditions, thus not present in most tests & extractions, but i wondered if some (or all) of the DMT in the plant (after basic extraction) is in fact derived from the yuremamine 'hyper-molecule'..as it is suspected of MAOI and psychoactivity, this could explain the traditional 'Jurema' drink made without MAOI plants having activity, if made with only cold water techniques..
thinking about how molecules could reside inside larger molecules, i came across two interesting references..
..according to S. Voogenbreinder's Garden Of Eden, Psychotria beccaroides (from New Guinea), P. forsteriana (Vanuata), and P. lyciiflora and P. oleoides (from New Caledonia) contain:
hodgkinsine is found in the south american Psychotria colorata (with tradtional medicinal usage), and has also been found to act as both a mu opioid agonist and an NMDA antagonist [Amador et al. "Antinociceptive profile of hodgkinsine" Planta Medica 2000 Dec.] the bioactivity, like the molecule, is comlplex..
wikipedia says:
this opens up a whole new world of cold-water extraction 'hyperloids' with all sorts of possible effects and possible manipulations,
& old world lost usages of the Jurema or 'Ajuca' sacred drink..
..not to mention ideas about how alkaloids transform and disguise themselves within plants
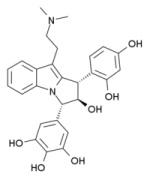
for more on yuremamine see:
Vespålåinen, J.J. et al. "Isolation and characterization of yuremamine, a new phytoindole." Planta Medica 71 1053-1057
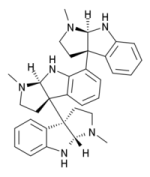
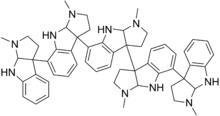
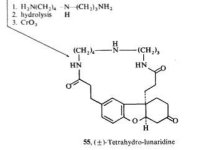
here are the structures of hodgkinsine, yuremamine & psychotridine:
in What are the current views, facts and theories on DMT-N-OXIDE? - DMT Discussion - Welcome to the DMT-Nexus(see posts #11 and #13 for detail and refs) .. endlessness noted that it contains within it a DMT molecule..it is broken down into constituents by heat and basic conditions, thus not present in most tests & extractions, but i wondered if some (or all) of the DMT in the plant (after basic extraction) is in fact derived from the yuremamine 'hyper-molecule'..as it is suspected of MAOI and psychoactivity, this could explain the traditional 'Jurema' drink made without MAOI plants having activity, if made with only cold water techniques..
thinking about how molecules could reside inside larger molecules, i came across two interesting references..
..according to S. Voogenbreinder's Garden Of Eden, Psychotria beccaroides (from New Guinea), P. forsteriana (Vanuata), and P. lyciiflora and P. oleoides (from New Caledonia) contain:
complex indole alkaloids called Psychotridines - NMT-derived alkaloids made by linking 2-8 pyrrolidinoindoline groups. They are potently cytotoxic against rat hepatoma & human leukaemia cell lines, inhibit platelet aggregation, and are antibacterial, strongly sedative and analgesic in mice (CSIRO 1990; Janic et al. 1999; Saad et al. 1995)
The Australian shrub Hodgkinsonia frutescens (Rutaceae) ..contains a complex tryptamine-derived alkaloid..called hodgkinsine. Given orally to mice, 25-50mg/kg produced mild sedation and loss of balance..
hodgkinsine is found in the south american Psychotria colorata (with tradtional medicinal usage), and has also been found to act as both a mu opioid agonist and an NMDA antagonist [Amador et al. "Antinociceptive profile of hodgkinsine" Planta Medica 2000 Dec.] the bioactivity, like the molecule, is comlplex..
wikipedia says:
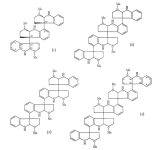
it should be noted that in bio-assay experiments the 'psychotropic effects' are either de-emphasised or hard to judge..'sedation' or 'loss of balance' in mice could mean a lot of possible different things..thankfully their medical value seems confirmed and growing..Hodgkinsine is a 'trimer' composed of three pyrrolidinoindoline subunits, with the monomer closely resembling another alkaloid eseroline which has similar bioactivity. Due to it's complex structure and multiple chiral centres, hodgkinsine has many stereoisomers and significant research has been undertaken to elucidate the structure-acticity relationships of the various isomers..
this opens up a whole new world of cold-water extraction 'hyperloids' with all sorts of possible effects and possible manipulations,
& old world lost usages of the Jurema or 'Ajuca' sacred drink..
..not to mention ideas about how alkaloids transform and disguise themselves within plants
for more on yuremamine see:
Vespålåinen, J.J. et al. "Isolation and characterization of yuremamine, a new phytoindole." Planta Medica 71 1053-1057
here are the structures of hodgkinsine, yuremamine & psychotridine: