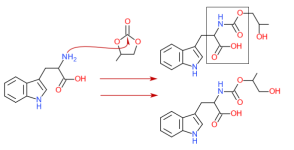
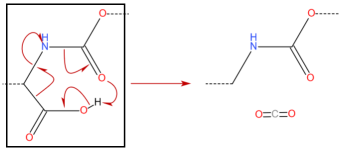
Hello,
as I was not able to find anything about this in the forum I will drop points of my theoretical thought process here. I am not a chemist so please bear with me
Intro:
I know esterification from steroids.
The complexity of the ester has impact on the half life.
From my knowledge the body has first to break down the ester before the substance is available.
As example testosterone propionate (20 hours half life) which has a low ester chain and
testosterone enanthate (4,5 days) which has a high ester chain.
Types of esters I am aware of: propionate, enanthate, acetate, cypionate.
I also think that the same concept is applied to some medications, I currently cannot think of any.
Intention:
Finding another sensible way to extend the journey.
Questions:
1. Would it be possible to apply esterification on psychoactive substances as well for oral or vaping use?
2. Does it make any sense?
3. Would it be safe to process and use?
4. I am aware of the tolerances but would it make even more sense to combine it with MAOIs?
References for chemical structures:
Propionate: Testosterone propionate - Wikipedia
Enanthate: Testosterone enanthate - Wikipedia
as I was not able to find anything about this in the forum I will drop points of my theoretical thought process here. I am not a chemist so please bear with me
Intro:
I know esterification from steroids.
The complexity of the ester has impact on the half life.
From my knowledge the body has first to break down the ester before the substance is available.
As example testosterone propionate (20 hours half life) which has a low ester chain and
testosterone enanthate (4,5 days) which has a high ester chain.
Types of esters I am aware of: propionate, enanthate, acetate, cypionate.
I also think that the same concept is applied to some medications, I currently cannot think of any.
Intention:
Finding another sensible way to extend the journey.
Questions:
1. Would it be possible to apply esterification on psychoactive substances as well for oral or vaping use?
2. Does it make any sense?
3. Would it be safe to process and use?
4. I am aware of the tolerances but would it make even more sense to combine it with MAOIs?
References for chemical structures:
Propionate: Testosterone propionate - Wikipedia
Enanthate: Testosterone enanthate - Wikipedia