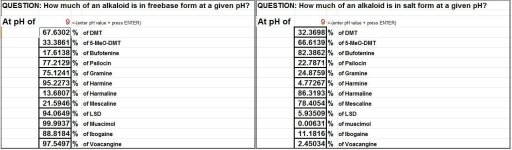
Hi all,
SWIM made a small Excel document (see snapshot) which calculates the percentage of an alkaloid that is in its freebase form at a certain pH given that its pKa is known. The applet is horrid since SWIM's not a programmer. Should anyone desires, feel free to develop a better applet for it.
This Excel programme is for mostly for curiosity and entertainment purposes. Playing around with it people can see how gradual increases/decreases of the pH affect the amount of alkaloids in freebase form.
But it can be a useful tool as well; say that someone wants to selectively precipitate harmine (pKa=7.7) out of harmaline(pKa=9.8 ) out of a solution. Which is the best pH value where he will precipitate most of the harmine out and the least amount of harmine?
SWIM plotter a graph showing freebase harmine depletion/precipitation (that equals harmine salt left in solution, blue line) versus freebase harmaline accumulatio (pink line).
The intersection point corresponds at a pH of around 8.75, which is the pH where one will get the most harmine with the least harmaline contamination. At this pH one will in theory get at around 92% harmine and 8% harmaline in his precipitates.
From the graph one can also see that at pH of around 8 one will get only minimal harmaline contamination, yet he will only precipitate roughly 68% of the harmine.
EDIT:
A small disclaimer for the use of these equations.
1) In theory, theory and practise are the same, but in practise they are not. That is to say that all these equations are purely theoretical. In real life things might be (and often are) a bit different.
2) The equations give a very simple result. Simply the amount of the alkaloid that exists in freebase and salt form. It does not imply anything about precipitation, solubility etc.
3) The results of these equations are more realistic the more pure the preparation of an alkaloid is. An alkaloid present in a crude solution with other crap may behave differently. A relatively pure alkaloid however is more likely to behave as the equations predict.
SWIM made a small Excel document (see snapshot) which calculates the percentage of an alkaloid that is in its freebase form at a certain pH given that its pKa is known. The applet is horrid since SWIM's not a programmer. Should anyone desires, feel free to develop a better applet for it.
This Excel programme is for mostly for curiosity and entertainment purposes. Playing around with it people can see how gradual increases/decreases of the pH affect the amount of alkaloids in freebase form.
But it can be a useful tool as well; say that someone wants to selectively precipitate harmine (pKa=7.7) out of harmaline(pKa=9.8 ) out of a solution. Which is the best pH value where he will precipitate most of the harmine out and the least amount of harmine?
SWIM plotter a graph showing freebase harmine depletion/precipitation (that equals harmine salt left in solution, blue line) versus freebase harmaline accumulatio (pink line).
The intersection point corresponds at a pH of around 8.75, which is the pH where one will get the most harmine with the least harmaline contamination. At this pH one will in theory get at around 92% harmine and 8% harmaline in his precipitates.
From the graph one can also see that at pH of around 8 one will get only minimal harmaline contamination, yet he will only precipitate roughly 68% of the harmine.
EDIT:
A small disclaimer for the use of these equations.
1) In theory, theory and practise are the same, but in practise they are not. That is to say that all these equations are purely theoretical. In real life things might be (and often are) a bit different.
2) The equations give a very simple result. Simply the amount of the alkaloid that exists in freebase and salt form. It does not imply anything about precipitation, solubility etc.
3) The results of these equations are more realistic the more pure the preparation of an alkaloid is. An alkaloid present in a crude solution with other crap may behave differently. A relatively pure alkaloid however is more likely to behave as the equations predict.