Nitegazer
Rising Star
I believe I have successfully reduced Harmaline to THH with the help of the great folks here at the Nexus-- special thanks to Endlessness and Tregar.
The good news here for Nexians is that I am not a chemist, so I think the process is suitably easy and safe for most here to try. Of course, safety practices such as protective eye-wear need to be followed, as a strong acid is used. Also note that the reaction releases hydrogen gas, so you will want to ventilate properly.
I will also say that I messed up a bit, adding too much HCl solution at the start, which necessitated much more ammonia to basify than would typically be needed. I also have questions for the more experienced folks out there that I will type in orange because of the season.
-----------------------------------------------------------------------------------------------
I began with 3g of harmaline, which was separated out of a clean rue freebase using sodium bicarbonate two times, to get it reasonably free of harmine.
Harmalas Extraction and Separation Guide
- I dissolved the 3g harmaline in 60ml of 32% HCl and 140ml of distilled water (200ml total). The solution had to be heated in a water bath and mixed for a while (5 minutes) in order for the harmaline to dissolve into a clear yellow solution. I think this is much more acid than should have been used, but would like confirmation of this. I did some back of the envelope calcs and think I should have only used about 36ml of the HCl.) Also, I'm surprised it was so hard to get the harmaline to dissolve-- would appreciate an insight
- While keeping the vessel at near boiling temp in a water heat bath, 12g of zinc dust was added a pinch at a time. There was a lot of fizzing and bubbling, and I would mix after each addition of zinc. I ended up using an additional 50ml of water to wash residue back into solution during the process.
- Kept stirring over the next hour, while the zinc fizzed away and danced in the solution (very fun to watch for a non-chemist, btw). At the end, the solution had gone nearly clear, with just a slight tint of yellow-green.
- Stirred in 40g of Ammonium Chloride in the still heated solution (AniMed for horses, cows and such, easy to find in the US). As I added the salt, something kept wanting to fall out of the solution. I added another 150 ml of water and kept stirring until it all remained dissolved (total solution volume now 400ml). Was this the THH or was it zinc chloride? Did I need to add the water or should I have just filtered the stuff out? I feel like this was a lot of solution for a just 3g of starting material.
- Ran through a filter (very little zinc remained, which is not surprising, given the excess of HCl). The solution was then basified with a F-ton of ammonia-- at least 700ml (I used ph papers to confirm I was getting the ph I needed). The resulting THH then quickly fell out of solution.
I wanted to test if this is really THH, but lacking any serious analytical equipment, I decided to use the melting point of THH (190C); harmaline has a melting point of 234C. I put 120mg in the oven and heated to 405 degrees f (207C). After 10 minutes nothing seemed to happen, so I raised the temp to 415 degrees f (213c).
Within minutes the color darkened, and I pulled it from the oven and scraped it from the dish it was in. It had a tar-like consistency that quickly hardened when cooled. It also had reduced in mass from 120mg to 100mg. I took this as confirmation that the reduction was at least a partial success, though I don't know the percentage of conversion.
The (assumed) THH has virtually no taste, with only a little lingering bitterness. Some have reported a metallic taste but I find none. I think this suggests little to no zinc salt contamination.
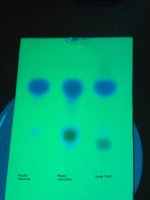
The resulting 2.5g of end product is in the first pic. The second pic is the result after heating (melting) in the oven to test the melting point.
The good news here for Nexians is that I am not a chemist, so I think the process is suitably easy and safe for most here to try. Of course, safety practices such as protective eye-wear need to be followed, as a strong acid is used. Also note that the reaction releases hydrogen gas, so you will want to ventilate properly.
I will also say that I messed up a bit, adding too much HCl solution at the start, which necessitated much more ammonia to basify than would typically be needed. I also have questions for the more experienced folks out there that I will type in orange because of the season.
-----------------------------------------------------------------------------------------------
I began with 3g of harmaline, which was separated out of a clean rue freebase using sodium bicarbonate two times, to get it reasonably free of harmine.
Harmalas Extraction and Separation Guide
- I dissolved the 3g harmaline in 60ml of 32% HCl and 140ml of distilled water (200ml total). The solution had to be heated in a water bath and mixed for a while (5 minutes) in order for the harmaline to dissolve into a clear yellow solution. I think this is much more acid than should have been used, but would like confirmation of this. I did some back of the envelope calcs and think I should have only used about 36ml of the HCl.) Also, I'm surprised it was so hard to get the harmaline to dissolve-- would appreciate an insight
- While keeping the vessel at near boiling temp in a water heat bath, 12g of zinc dust was added a pinch at a time. There was a lot of fizzing and bubbling, and I would mix after each addition of zinc. I ended up using an additional 50ml of water to wash residue back into solution during the process.
- Kept stirring over the next hour, while the zinc fizzed away and danced in the solution (very fun to watch for a non-chemist, btw). At the end, the solution had gone nearly clear, with just a slight tint of yellow-green.
- Stirred in 40g of Ammonium Chloride in the still heated solution (AniMed for horses, cows and such, easy to find in the US). As I added the salt, something kept wanting to fall out of the solution. I added another 150 ml of water and kept stirring until it all remained dissolved (total solution volume now 400ml). Was this the THH or was it zinc chloride? Did I need to add the water or should I have just filtered the stuff out? I feel like this was a lot of solution for a just 3g of starting material.
- Ran through a filter (very little zinc remained, which is not surprising, given the excess of HCl). The solution was then basified with a F-ton of ammonia-- at least 700ml (I used ph papers to confirm I was getting the ph I needed). The resulting THH then quickly fell out of solution.
I wanted to test if this is really THH, but lacking any serious analytical equipment, I decided to use the melting point of THH (190C); harmaline has a melting point of 234C. I put 120mg in the oven and heated to 405 degrees f (207C). After 10 minutes nothing seemed to happen, so I raised the temp to 415 degrees f (213c).
Within minutes the color darkened, and I pulled it from the oven and scraped it from the dish it was in. It had a tar-like consistency that quickly hardened when cooled. It also had reduced in mass from 120mg to 100mg. I took this as confirmation that the reduction was at least a partial success, though I don't know the percentage of conversion.
The (assumed) THH has virtually no taste, with only a little lingering bitterness. Some have reported a metallic taste but I find none. I think this suggests little to no zinc salt contamination.
The resulting 2.5g of end product is in the first pic. The second pic is the result after heating (melting) in the oven to test the melting point.