Mescaline salts are very interesting. In particular, the sulfate salt 2(MescalineH)SO4 is soluble in hot water, but only slight soluble in cold water. Also, some people have reported that they can extract the natural salt with warm IPA. These extractions result in active resins after drying from what I have read, please correct me if this is wrong.
Mescaline HCl has low solubility in cold 99% IPA/Acetone (some discussion here).
The difference salt behavior is interesting. Why couldn't one pull these natural (more soluble?) MescalineH+ salts in acetone/IPA, then introduce the SO4-- or Cl- ions to see if things crash?
Someone must have tried doing a 99% or 91% IPA pull followed by cold and/or adding HCl. Do the actives crash in this case? I've searched the nexus and the net for this discussion but haven't found it (sometimes people don't report on negative results though).
The case of a warm acetone acetone pull followed by adding and SO4-- is a little more tricky, has anyone worked on this? A relatively neutral SO4-- salt would need to be added with water (to keep pH stable and avoid the more soluble and more acidic (MescalineH)HSO4). Water needs to be added with the salt because as far as I know there are no SO4-- salts soluble in acetone. Also, care must be taken to stay in the one phase region of the acetone/water/salt terniary system (sometimes represented by a gibbs triangle).
It would go something like this for acetone,
1) Extract cactus powder with warm acetone
2) Add X% water to the acetone extract so it can receive neutral SO4-- ions
3) Slowly add Y% salt solution (MgSO4 or (NH4)2SO4) and see if Mescaline sulfate clouds form in cold conditions.
X% and Y% are determined by the gibbs triangle for example. I can't find this triangle for the acetone/water/(MgSO4 or (NH4)2SO4) systems (if anyone can find it I would appreciate it). One can do tests with plain water/acetone and salt solutions. Want to add the max salt without clouds forming or layers separating.
I found the Gibbs triangle for IPA/water/NaCl, but since we could use HCl to test for precious there that is not as interesting. It does suggest that X% = 30 and Y% = 5 (or even Y=15%) would work (keeping the solution safely in the single phase region towards the bottom left as the salt solution is added) but one could be more aggressive (see image below)
Any thoughts? Seems like if this worked out would have been discussed already (?)
Mescaline HCl has low solubility in cold 99% IPA/Acetone (some discussion here).
The difference salt behavior is interesting. Why couldn't one pull these natural (more soluble?) MescalineH+ salts in acetone/IPA, then introduce the SO4-- or Cl- ions to see if things crash?
Someone must have tried doing a 99% or 91% IPA pull followed by cold and/or adding HCl. Do the actives crash in this case? I've searched the nexus and the net for this discussion but haven't found it (sometimes people don't report on negative results though).
The case of a warm acetone acetone pull followed by adding and SO4-- is a little more tricky, has anyone worked on this? A relatively neutral SO4-- salt would need to be added with water (to keep pH stable and avoid the more soluble and more acidic (MescalineH)HSO4). Water needs to be added with the salt because as far as I know there are no SO4-- salts soluble in acetone. Also, care must be taken to stay in the one phase region of the acetone/water/salt terniary system (sometimes represented by a gibbs triangle).
It would go something like this for acetone,
1) Extract cactus powder with warm acetone
2) Add X% water to the acetone extract so it can receive neutral SO4-- ions
3) Slowly add Y% salt solution (MgSO4 or (NH4)2SO4) and see if Mescaline sulfate clouds form in cold conditions.
X% and Y% are determined by the gibbs triangle for example. I can't find this triangle for the acetone/water/(MgSO4 or (NH4)2SO4) systems (if anyone can find it I would appreciate it). One can do tests with plain water/acetone and salt solutions. Want to add the max salt without clouds forming or layers separating.
I found the Gibbs triangle for IPA/water/NaCl, but since we could use HCl to test for precious there that is not as interesting. It does suggest that X% = 30 and Y% = 5 (or even Y=15%) would work (keeping the solution safely in the single phase region towards the bottom left as the salt solution is added) but one could be more aggressive (see image below)
Any thoughts? Seems like if this worked out would have been discussed already (?)


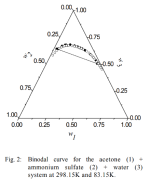
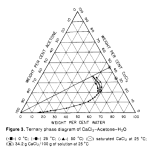
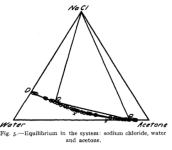
 . MgSO4 is not soluble in acetone, so it may not be as useful as malic acid for the cleaned up solution that has acetone added, but who knows: the SO4-- ion is special for
. MgSO4 is not soluble in acetone, so it may not be as useful as malic acid for the cleaned up solution that has acetone added, but who knows: the SO4-- ion is special for 