Hi all,
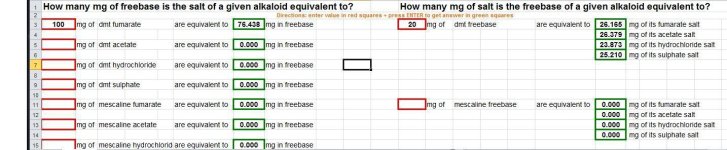
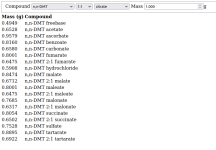
Ever had to calculate how much freebase a given alkaloid salt equals to? Or how much of a salt a freebase should make? Then look no further! Please find attached a simple excel calculator that easily converts the between salts and freebases for the celebrity alkaloids and their salts around here,
Cheers,
Infun
Ever had to calculate how much freebase a given alkaloid salt equals to? Or how much of a salt a freebase should make? Then look no further! Please find attached a simple excel calculator that easily converts the between salts and freebases for the celebrity alkaloids and their salts around here,
Cheers,
Infun