Salvinorin A is the active principle in the leaves of the psychotropic mint Salvia divinorum. Salvinorin A is a pharmacologically interesting compound; it is a kappa opioid receptor (KOR) agonist and as such it exerts its effects in a different way compared to the other classical psychedelics that act primarily on serotonin receptors. More intriguing though, it is possibly the first non-nitrogen containing molecule with psychedelic properties. It is not an alkaloid but a diterpene. Despite its unique psychedelic properties and remarkable potency, there have been limited (but thankfully insightful) investigations on its mechanism of action, metabolism and pharmacokinetics.
Here I summarise some important aspect on Salvinorins structure and how their structure affects their action. I also discuss the metabolism of Salvinorin A and how it may be affected by pharmacologican intervention. I also present data on some Salvinorin analogues and finally, I speculate on possible future directions on this very promising field of psychedelic substrances.
Salvinorins: What makes them active or inactive?
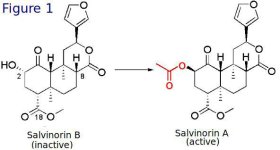
As mentioned above, Salvinorin A is a potent KOR agonist. Salvinorin B on the other hand is a very weak KOR agonist and is thought not to have psychedelic qualities. Salvinorin A and B are remarkably similar in structure (see Figure 1). Their difference is the addition of an acetate ester group (shown in red) on the carbon position 2. Technically speaking, Salvinorin A is the acetate ester of Salvinorin B. It has been demonstrated using molecular modeling on receptor-ligand interaction of Salvinorin A and KOR that this acetate ester is critical for their stabilisation of their interaction. Salvinorin B on the other hand lacks this group and it is a very weak KOR agonist.
The dramatic increase in potency brought by by the addition of the acetate ester on the Salvinorin molecule opens up new horizons and creates many questions. To the author's interest lie the putative metabolism of Salvinorin A in the body as well as the potential to synthesize novel analogues of Salvinorin B so as to create active Salvinorins with altered properties.
Uptake and clearance of Salvinorin A.
Salvinorin A is active either by smoking or absorption from the cavity of the mouth. In either case, it produces a short-lived experience which ranges from 5-10 min (when smoked) to almost an hour (when leaves are chewed). Both of these common methods of administration aim at the delivery of Salvinorin from the lungs (smoked) or the mouth cavity (chewed) to the blood from where it can reach the brain and exert its actions.
Salvinorin A is reported to be not orally active. The latter statement is partially true however and Salvinorin has been reported to be orally active but much larger quantities reaching 20-25 times more than smoked need to be ingested.
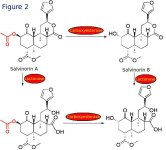
So, what makes Salvinorin A inactive when taken orally? Or likewise, which mechanism is responsible for the end of the experience after smoking or chewing Salvia leaves? A very recent publication showed that Salvinorin A is rapidly degraded in the blood (see attached abstract from Tsujikawa et al). This group showed that Salvinorin A is degraded to its deacetylated inactive form (which is Salvinorin B) by a group of enzymes called esterases. Using pharmacological intervention using esterase inhibitors they found that sodium fluoride, phenylmethylsulfonyl fluoride and bis-p-nitrophenyl phosphate (all of them are esterase inhibitors) increased markedly the stability of Salvinorin A. A few more experiments showed that the most likely enzyme responsible for the deacetylation of Salvinorin A is a carboxylesterase.
The same group also identified another degradation product of Salvinorin A in the blood. This by-product had a lactone-open ring and this is most likely due to the action of a lactonase enzyme. The degradation pathways of Salvinorin A are summarised in Figure 2.
Degradation of Salvinorin A to B and/or destruction of its lactone ring has also been suggested to be the likely mechanism that marks the end of its action on the body. The (not mutually exclusive) KOR receptor desensitisation, also known as tolerance building, is another plausible mechanism that may account for the end of Salvinorin A's effects. There are not enough data to support the latter however. On the contrary, synthetic Salvinorin analogues that have the ability to bind and activate the KOR receptor but cannot be hydrolysed by carboxylesterases have longer-lasting action. This suggests that deacetylation of Salvinorin a to B is the prime mechanism that limits its action.
Salvia divinorum analogues
There have been research groups that have managed to synthesise analogues of Salvinorin A. The interest in synthesizing synthetic analogues is manifold; analogues can be much more potent, more longer-lasting, more specific, more stable and they can have different qualities (e.g. they can block KOR instead of activating it). For a not exhaustive list of Salvinorin analogues see the attached paper from Beguin et al.
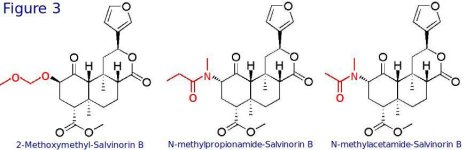
The most interesting Salvinorinn analogues are the ones that have substitution on the C2 carbon of Salvinorin B, i.e. the substitutions that confer binding of Salvinorins on the KOR receptor and/or remove the carboxyesterase-sensitive acetate ester. The compounds 2-Methoxymethyl-Salvinorin B, N-methylpropionamide Salvinorin B and N-methylacetamide Salvinorin B (Fig 3) are the most promising and their properties, differences and structures will be discussed below:
2-Methoxymethyl-Salvinorin B (MOM)
This compound is similar to Salvinorin A but has a methoxymethyl ether group instead of an acetate ester bond. It is reported to have a 3-fold higher affinity on the human KOR receptor compared to Salvinorin A and is therefore more potent than Salvinorin A. Intraperitoneal injection of this compound in mice and rats has shown 5- to 6-fold duration of the experience. The much longer lasting effects are thought to be a result of the inability of carboxylesterases to degrade MOM to the inactive Salvinorin B. There have not been reports of its effects in humans. For more information see the paper from Wang et al attached below.
N-methylpropionamide Salvinorin B (MPA)]
Synthesis of this compound involves conversion of Salvinorin B to its derivative n-methyl amine and then forming an amide bond this n-methyl amine derivative and proprionic acid. The result is actually a Salvinorin analogue that also contains a nitrogen atom, which technically speaking makes it an alkaloid. In contrast to MOM, MPA is not more potent than Salvinorin, it is however 10 times longer active than Salvinorin A. Again, its much longer-lasting action is thought to be due to the inability of the carboxylesterases to attack the molecule. There have not been tests of this compound in humans.
N-methylacetamide Salvinorin B (MNA)
Almost just like MPA, MNA is the amide formed by the reaction of an n-methyl amine derivative of Salvinorin B and acetic acid. Its action is very similar in many respects to MPA; it is no more potent than Salvinorin A and it lasts around 6 times as much as Salvinorin A. The most interesting fact about this compound is that contrary to MOM and MPA, it is orally active (at least in rats). Again, there have not been tests of this compound in humans. For more information on both MPA and MNA see the paper from Beguin et al, attached below.
The fact that MNA is orally active whereas MOM and MPA are not is intriguing. There is at least another enzyme that metabolises Salvinorins. Lactonase has been shown to degrade Salvinorins (Tsujikawa et al) in the blood. I speculate that a lactonase enzyme also degrades Salvinorins in the digestive tract. In this respect, MNA may be a very poor substrate or even inhibitor of this enzyme.
The Future of Psychedelic Research on Salvinorins.
The researrch on Salvinorin analogues is still in its infancy and there are still many uncharted areas. Research groups have managed to alter the chemical structure of Salvinorins found in S.divinorum to create compounds that are more potent, longer lasting and with expanded administration routes compared to Salvinorin A. This can be thought analogous to the development of LSD from lysergic derivatives of ergot alkaloids. It is very unfortunate that no reports can be found on the use of these novel Salvinorin analogues in humans. I feel that some day people will approach these (and future) compound and will be able to appreciate whether these compounds have different/better/worse/more insightful/less insightful etc. qualities than Salvinorin A.
More importantly though, Salvinorin analogues are (and will continue to to be) useful tools in the investigation of the mechanism of action and metabolism of Salvinorin A. Should we reach the equivalent level of understanding we currently have for the MAO system and psychedelic tryptamines, we may be able to prepare plant or pharmacological admixtures to the current S.divinorum preparations so as to control or direct the effects of Salvinorin A. In theory therefore, we could get to control the qualities of the Salvinorin A experience (e.g. make it more/less visual, more/less weird, more recreational, more insightful etc). Even the possibility of orally administering S.divinorum is open if in theory it is combined with certain esterases.
Here I summarise some important aspect on Salvinorins structure and how their structure affects their action. I also discuss the metabolism of Salvinorin A and how it may be affected by pharmacologican intervention. I also present data on some Salvinorin analogues and finally, I speculate on possible future directions on this very promising field of psychedelic substrances.
Salvinorins: What makes them active or inactive?
As mentioned above, Salvinorin A is a potent KOR agonist. Salvinorin B on the other hand is a very weak KOR agonist and is thought not to have psychedelic qualities. Salvinorin A and B are remarkably similar in structure (see Figure 1). Their difference is the addition of an acetate ester group (shown in red) on the carbon position 2. Technically speaking, Salvinorin A is the acetate ester of Salvinorin B. It has been demonstrated using molecular modeling on receptor-ligand interaction of Salvinorin A and KOR that this acetate ester is critical for their stabilisation of their interaction. Salvinorin B on the other hand lacks this group and it is a very weak KOR agonist.
The dramatic increase in potency brought by by the addition of the acetate ester on the Salvinorin molecule opens up new horizons and creates many questions. To the author's interest lie the putative metabolism of Salvinorin A in the body as well as the potential to synthesize novel analogues of Salvinorin B so as to create active Salvinorins with altered properties.
Uptake and clearance of Salvinorin A.
Salvinorin A is active either by smoking or absorption from the cavity of the mouth. In either case, it produces a short-lived experience which ranges from 5-10 min (when smoked) to almost an hour (when leaves are chewed). Both of these common methods of administration aim at the delivery of Salvinorin from the lungs (smoked) or the mouth cavity (chewed) to the blood from where it can reach the brain and exert its actions.
Salvinorin A is reported to be not orally active. The latter statement is partially true however and Salvinorin has been reported to be orally active but much larger quantities reaching 20-25 times more than smoked need to be ingested.
So, what makes Salvinorin A inactive when taken orally? Or likewise, which mechanism is responsible for the end of the experience after smoking or chewing Salvia leaves? A very recent publication showed that Salvinorin A is rapidly degraded in the blood (see attached abstract from Tsujikawa et al). This group showed that Salvinorin A is degraded to its deacetylated inactive form (which is Salvinorin B) by a group of enzymes called esterases. Using pharmacological intervention using esterase inhibitors they found that sodium fluoride, phenylmethylsulfonyl fluoride and bis-p-nitrophenyl phosphate (all of them are esterase inhibitors) increased markedly the stability of Salvinorin A. A few more experiments showed that the most likely enzyme responsible for the deacetylation of Salvinorin A is a carboxylesterase.
The same group also identified another degradation product of Salvinorin A in the blood. This by-product had a lactone-open ring and this is most likely due to the action of a lactonase enzyme. The degradation pathways of Salvinorin A are summarised in Figure 2.
Degradation of Salvinorin A to B and/or destruction of its lactone ring has also been suggested to be the likely mechanism that marks the end of its action on the body. The (not mutually exclusive) KOR receptor desensitisation, also known as tolerance building, is another plausible mechanism that may account for the end of Salvinorin A's effects. There are not enough data to support the latter however. On the contrary, synthetic Salvinorin analogues that have the ability to bind and activate the KOR receptor but cannot be hydrolysed by carboxylesterases have longer-lasting action. This suggests that deacetylation of Salvinorin a to B is the prime mechanism that limits its action.
Salvia divinorum analogues
There have been research groups that have managed to synthesise analogues of Salvinorin A. The interest in synthesizing synthetic analogues is manifold; analogues can be much more potent, more longer-lasting, more specific, more stable and they can have different qualities (e.g. they can block KOR instead of activating it). For a not exhaustive list of Salvinorin analogues see the attached paper from Beguin et al.
The most interesting Salvinorinn analogues are the ones that have substitution on the C2 carbon of Salvinorin B, i.e. the substitutions that confer binding of Salvinorins on the KOR receptor and/or remove the carboxyesterase-sensitive acetate ester. The compounds 2-Methoxymethyl-Salvinorin B, N-methylpropionamide Salvinorin B and N-methylacetamide Salvinorin B (Fig 3) are the most promising and their properties, differences and structures will be discussed below:
2-Methoxymethyl-Salvinorin B (MOM)
This compound is similar to Salvinorin A but has a methoxymethyl ether group instead of an acetate ester bond. It is reported to have a 3-fold higher affinity on the human KOR receptor compared to Salvinorin A and is therefore more potent than Salvinorin A. Intraperitoneal injection of this compound in mice and rats has shown 5- to 6-fold duration of the experience. The much longer lasting effects are thought to be a result of the inability of carboxylesterases to degrade MOM to the inactive Salvinorin B. There have not been reports of its effects in humans. For more information see the paper from Wang et al attached below.
N-methylpropionamide Salvinorin B (MPA)]
Synthesis of this compound involves conversion of Salvinorin B to its derivative n-methyl amine and then forming an amide bond this n-methyl amine derivative and proprionic acid. The result is actually a Salvinorin analogue that also contains a nitrogen atom, which technically speaking makes it an alkaloid. In contrast to MOM, MPA is not more potent than Salvinorin, it is however 10 times longer active than Salvinorin A. Again, its much longer-lasting action is thought to be due to the inability of the carboxylesterases to attack the molecule. There have not been tests of this compound in humans.
N-methylacetamide Salvinorin B (MNA)
Almost just like MPA, MNA is the amide formed by the reaction of an n-methyl amine derivative of Salvinorin B and acetic acid. Its action is very similar in many respects to MPA; it is no more potent than Salvinorin A and it lasts around 6 times as much as Salvinorin A. The most interesting fact about this compound is that contrary to MOM and MPA, it is orally active (at least in rats). Again, there have not been tests of this compound in humans. For more information on both MPA and MNA see the paper from Beguin et al, attached below.
The fact that MNA is orally active whereas MOM and MPA are not is intriguing. There is at least another enzyme that metabolises Salvinorins. Lactonase has been shown to degrade Salvinorins (Tsujikawa et al) in the blood. I speculate that a lactonase enzyme also degrades Salvinorins in the digestive tract. In this respect, MNA may be a very poor substrate or even inhibitor of this enzyme.
The Future of Psychedelic Research on Salvinorins.
The researrch on Salvinorin analogues is still in its infancy and there are still many uncharted areas. Research groups have managed to alter the chemical structure of Salvinorins found in S.divinorum to create compounds that are more potent, longer lasting and with expanded administration routes compared to Salvinorin A. This can be thought analogous to the development of LSD from lysergic derivatives of ergot alkaloids. It is very unfortunate that no reports can be found on the use of these novel Salvinorin analogues in humans. I feel that some day people will approach these (and future) compound and will be able to appreciate whether these compounds have different/better/worse/more insightful/less insightful etc. qualities than Salvinorin A.
More importantly though, Salvinorin analogues are (and will continue to to be) useful tools in the investigation of the mechanism of action and metabolism of Salvinorin A. Should we reach the equivalent level of understanding we currently have for the MAO system and psychedelic tryptamines, we may be able to prepare plant or pharmacological admixtures to the current S.divinorum preparations so as to control or direct the effects of Salvinorin A. In theory therefore, we could get to control the qualities of the Salvinorin A experience (e.g. make it more/less visual, more/less weird, more recreational, more insightful etc). Even the possibility of orally administering S.divinorum is open if in theory it is combined with certain esterases.
Attachments
-
 salvinorins..jpg22.1 KB · Views: 0
salvinorins..jpg22.1 KB · Views: 0 -
 salvinorin+degradation+pathways..jpg46.9 KB · Views: 0
salvinorin+degradation+pathways..jpg46.9 KB · Views: 0 -
 salvinorin+analogues..jpg33.4 KB · Views: 0
salvinorin+analogues..jpg33.4 KB · Views: 0 -
2-Methoxymethyl-Salvinorin+B.pdf581.8 KB · Views: 0
-
N-Methylacetamide+analogue+of+Salvinorin.pdf418.1 KB · Views: 0
-
Pharmacokinetics+of+Salvinorin+A.pdf353.2 KB · Views: 0
-
Salvinorin+A+metabolism.txt1.8 KB · Views: 0
-
From+Natural+Products+to+Human+Therapeutics+.pdf861 KB · Views: 0
