-
Members of the previous forum can retrieve their temporary password here, (login and check your PM).
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
substituted phenethylamines/amphetamines: numbered counter-clockwise or clockwise, and why?
- Thread starter entheogenic-gnosis
- Start date
Migrated topic.
entheogenic-gnosis
Rising Star
I've been having issues with the two numbering systems and depictions for substituted phenethylamines/amphetamines, and wanted to hear others thoughts, but I think I solved it...
I've been rotating these molecules on their axis, and yes, as the molecules are non-planar, when rotated on their axis both depictions would appear correct depending on how the molecule is oriented in space...but this does not mean the methoxy groups are "switching" carbon atoms, or that carbons 2 and 5 and 3 and 6 are the same thing, which is how it kept getting explained to me...
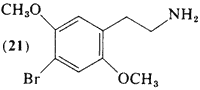
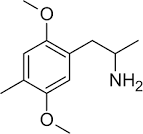
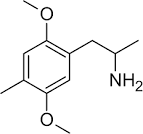
surly there must be some way to distinguish the orientations in space of 2,5-dimethoxy-4-substituted-amphetamines/phenethylamines from 3,6-dimethoxy-4-substituted-phenethylamines/amphetamines, right?
... and you would figure a single orientation and depiction of 2,5-dimethoxy-4-substituted-phenethylamines would be preferred, it's bad chemistry otherwise, because in this case 3,6-dimethoxy-4-x-phenethylamine/amphetamine and one orientation of 2,5-dimethoxy-4-x-phenethylamine/amphetamine would be depicted exactly the same way.
It's got to be the double bonds in the benzene ring, right?
Here, in the picture posted, pay attention to the double bonds, this is how the first two depictions can be showing the same molecule, and how the last molecule is representing 3,6-dimethoxy-4-x-amphetamine right?
It's got to be the damn double bonds, right?
If not, then please explain...
This can't be as complicated as I'm making it, since I left school I have had to teach myself, my friends and family know nothing about chemistry and don't understand or really seem to want to understand it, and the internet can't always supply a straight answer...
-eg
I've been rotating these molecules on their axis, and yes, as the molecules are non-planar, when rotated on their axis both depictions would appear correct depending on how the molecule is oriented in space...but this does not mean the methoxy groups are "switching" carbon atoms, or that carbons 2 and 5 and 3 and 6 are the same thing, which is how it kept getting explained to me...
surly there must be some way to distinguish the orientations in space of 2,5-dimethoxy-4-substituted-amphetamines/phenethylamines from 3,6-dimethoxy-4-substituted-phenethylamines/amphetamines, right?
... and you would figure a single orientation and depiction of 2,5-dimethoxy-4-substituted-phenethylamines would be preferred, it's bad chemistry otherwise, because in this case 3,6-dimethoxy-4-x-phenethylamine/amphetamine and one orientation of 2,5-dimethoxy-4-x-phenethylamine/amphetamine would be depicted exactly the same way.
It's got to be the double bonds in the benzene ring, right?
Here, in the picture posted, pay attention to the double bonds, this is how the first two depictions can be showing the same molecule, and how the last molecule is representing 3,6-dimethoxy-4-x-amphetamine right?
It's got to be the damn double bonds, right?
If not, then please explain...
This can't be as complicated as I'm making it, since I left school I have had to teach myself, my friends and family know nothing about chemistry and don't understand or really seem to want to understand it, and the internet can't always supply a straight answer...
-eg
Attachments
entheogenic-gnosis
Rising Star
Hmmm...
For some reason that doesn't sit well with me either though...even though it's the one of the best explanations yet...
What is the deal here?
-eg
For some reason that doesn't sit well with me either though...even though it's the one of the best explanations yet...
What is the deal here?
-eg
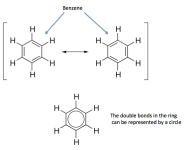
because its arbitrary. (and so are the double bonds, by the way, you can draw them in two different ways, both are correct, they exhibit resonance, it is more like 6 bonds that are inbetween single and double, the electrons are shared throughout the ring, than 3 double bonds, this is why you see phenyl rings sometimes with a circle inside instead of the 3 double bonds).
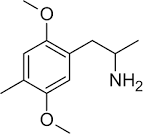
If there are no substituents you can number the carbons in either direction. The numbering only becomes standardized if there are substituents. In that case you number around the ring closest to the first substituent. For example, 2-methoxyphenethylamine , is the proper name, 6-methoxyphenethylamine is wrong. So following the same logic, 2,5-dimethoxy-4-bromophenethylamine is correct, 3,6-dimethoxy-4-bromophenethylamine is wrong. Don't use bigger numbers 3 and 6 over 2 and 5 when you don't have to. (They are, by the way, if you haven't noticed, the same molecule.. just mirror image..)
---
Edit:
here I attached an informative picture. Remember that the structural line drawings are just like a langauge to communicate structure to other chemists, and in no way describe the real shape or structure of the molecules.
Here is a picture showing benzene. The first two are only showing localized pi bonds. They only really exist like this (for any tangible length of time) if there is no conjugation. The 3rd picture (c) depicts how it truly exists in nature. It's double bonds are conjugated, so the electrons are delocalized throughout the pi-cloud.



If there are no substituents you can number the carbons in either direction. The numbering only becomes standardized if there are substituents. In that case you number around the ring closest to the first substituent. For example, 2-methoxyphenethylamine , is the proper name, 6-methoxyphenethylamine is wrong. So following the same logic, 2,5-dimethoxy-4-bromophenethylamine is correct, 3,6-dimethoxy-4-bromophenethylamine is wrong. Don't use bigger numbers 3 and 6 over 2 and 5 when you don't have to. (They are, by the way, if you haven't noticed, the same molecule.. just mirror image..)
---
Edit:
here I attached an informative picture. Remember that the structural line drawings are just like a langauge to communicate structure to other chemists, and in no way describe the real shape or structure of the molecules.
Here is a picture showing benzene. The first two are only showing localized pi bonds. They only really exist like this (for any tangible length of time) if there is no conjugation. The 3rd picture (c) depicts how it truly exists in nature. It's double bonds are conjugated, so the electrons are delocalized throughout the pi-cloud.
downwardsfromzero
Boundary condition
EG - The benzene ring spins relative to the aminopropyl side chain. The atoms themselves do not have numbers on. Did you have a look at the videos on the other thread? Unfortunately I can't show animated rotation within the molecule (yet) but keep looking and hopefully it will click for you.
Edit: it appears mindlusion posted a reply while I was still composing mine. Is this really a misunderstanding based on thinking that the benzene ring has fixed double bonds? In 2.5 years of o. chem they didn't teach you about the delocalised pi-bonds in the benzene ring?
Edit: it appears mindlusion posted a reply while I was still composing mine. Is this really a misunderstanding based on thinking that the benzene ring has fixed double bonds? In 2.5 years of o. chem they didn't teach you about the delocalised pi-bonds in the benzene ring?
If your double bonds were fixed, then your argument would apply. This seems to be the source of the misunderstanding. mindlusion's illustration (the third one) should clear this up.mindlusion said:the double bonds, by the way, you can draw them in two different ways, both are correct, they exhibit resonance
downwardsfromzero
Boundary condition
Some old chemistry books have the benzene ring depicted with a central circle and no double bonds to show the delocalised pi-bonds. This has been abandoned as it makes it too difficult to draw electron transfers, e.g. when depicting electrophilic aromatic substitution.
entheogenic-gnosis
Rising Star
I understand what you guys are saying, which is all correct, however I feel perhaps I'm the one being unclear..
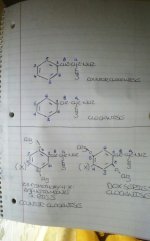
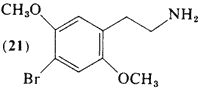
First, the double bonds in the benzene ring, see the picture below.
Watch the double bonds when you rotate a 3-d 2,5-dimethoxy-amphetamine molecule in 3-d. Just as the methoxy groups appear to have changed orientation, so have the double bonds.
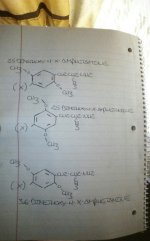
Next, there are 6 available carbon atoms, each with a number relative to the ethylamine chain at position 1, let's go through here
·Carbon 1 = ethylamine side chain
·Carbon 2 = methoxy group
·Carbon 3 = unsplit aromatic hydrogen atom
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = methoxy group
·Carbon 6 = unsplit aromatic hydrogen atom
Now say you want to depict this molecule:
·Carbon 1 = ethylamine side chain
·Carbon 2 = unsplit aromatic hydrogen atom
·Carbon 3 = methoxy group
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = unsplit aromatic hydrogen atom
·Carbon 6 = methoxy group
It's depiction would look identical to one of the orientations of 2,5-dimethoxy-4-x-phenethylamine...which can be depicted in two distinct orientations...
The only way to differentiate my proposed 3,6-dimethoxy-4-x-phenethylamine would be to alter the orientation of the double bonds in the benzene rings!
Seriously, in the other thread I was not listening, and probably looked like an s as, or perhaps just a bit rude, I apologize for that, but seriously, I finally think I git it! Look at the double bonds in relation to the ethylamine side chain when "flipping" the 3-d molecule.
Generally this wouldn't be an issue, but in order to distinguish the two 2-D orientations of 2,5-dimethoxy-4-x-phenethylamines from the 2-D depiction of 3,6-dimethoxy-4-x-phenethylamines.
-eg
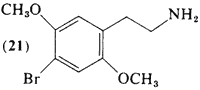
First, the double bonds in the benzene ring, see the picture below.
Watch the double bonds when you rotate a 3-d 2,5-dimethoxy-amphetamine molecule in 3-d. Just as the methoxy groups appear to have changed orientation, so have the double bonds.
Next, there are 6 available carbon atoms, each with a number relative to the ethylamine chain at position 1, let's go through here
·Carbon 1 = ethylamine side chain
·Carbon 2 = methoxy group
·Carbon 3 = unsplit aromatic hydrogen atom
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = methoxy group
·Carbon 6 = unsplit aromatic hydrogen atom
Now say you want to depict this molecule:
·Carbon 1 = ethylamine side chain
·Carbon 2 = unsplit aromatic hydrogen atom
·Carbon 3 = methoxy group
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = unsplit aromatic hydrogen atom
·Carbon 6 = methoxy group
It's depiction would look identical to one of the orientations of 2,5-dimethoxy-4-x-phenethylamine...which can be depicted in two distinct orientations...
The only way to differentiate my proposed 3,6-dimethoxy-4-x-phenethylamine would be to alter the orientation of the double bonds in the benzene rings!
Seriously, in the other thread I was not listening, and probably looked like an s as, or perhaps just a bit rude, I apologize for that, but seriously, I finally think I git it! Look at the double bonds in relation to the ethylamine side chain when "flipping" the 3-d molecule.
Generally this wouldn't be an issue, but in order to distinguish the two 2-D orientations of 2,5-dimethoxy-4-x-phenethylamines from the 2-D depiction of 3,6-dimethoxy-4-x-phenethylamines.
-eg
Attachments
entheogenic-gnosis
Rising Star
entheogenic-gnosis said:Watch the double bonds when you rotate a 3-d 2,5-dimethoxy-amphetamine molecule in 3-d. Just as the methoxy groups appear to have changed orientation, so have the double bonds.
Next, there are 6 available carbon atoms, each with a number relative to the ethylamine chain at position 1, let's go through here
·Carbon 1 = ethylamine side chain
·Carbon 2 = methoxy group
·Carbon 3 = unsplit aromatic hydrogen atom
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = methoxy group
·Carbon 6 = unsplit aromatic hydrogen atom
Now say you want to depict this molecule:
·Carbon 1 = ethylamine side chain
·Carbon 2 = unsplit aromatic hydrogen atom
·Carbon 3 = methoxy group
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = unsplit aromatic hydrogen atom
·Carbon 6 = methoxy group
It's depiction would look identical to one of the orientations of 2,5-dimethoxy-4-x-phenethylamine...which can be depicted in two distinct orientations...
The only way to differentiate my proposed 3,6-dimethoxy-4-x-phenethylamine would be to alter the orientation of the double bonds in the benzene rings!
You are still not listening though, though the concept can be tough to understand at first. You have to throw out your old ideas first.
There is absolutely no difference between your proposed molecule and the actual one.
The double bonds do not have an orientation. It is an aromatic ring. You can think of it as a quantum effect if you like (because it is), the bonds are in BOTH orientations simultaneously.
Draw a circle inside the aromatic ring, then it might help you see that your two molecules are really exactly the same molecule. You are just flipping it to the mirror image. (and we are not talking about chirality here, obviously) The only thing different about it is the name you choose but it is incorrect nomenclature because you number in the wrong direction.
What you could call it is the alternate resonance structure, but even the resonance structures are identical, chemically speaking. Say if you did an EAS or NAS reaction it may prefer one or the other (just barely though), could use computational studies to figure that out if you wanted

entheogenic-gnosis
Rising Star
downwardsfromzero said:EG - The benzene ring spins relative to the aminopropyl side chain. The atoms themselves do not have numbers on. Did you have a look at the videos on the other thread? Unfortunately I can't show animated rotation within the molecule (yet) but keep looking and hopefully it will click for you.
Edit: it appears mindlusion posted a reply while I was still composing mine. Is this really a misunderstanding based on thinking that the benzene ring has fixed double bonds? In 2.5 years of o. chem they didn't teach you about the delocalised pi-bonds in the benzene ring?
If your double bonds were fixed, then your argument would apply. This seems to be the source of the misunderstanding. mindlusion's illustration (the third one) should clear this up.mindlusion said:the double bonds, by the way, you can draw them in two different ways, both are correct, they exhibit resonance
I know the double bonds are not fixed the in a benzene ring, I'm saying the orientation of the double bonds relative to the ethylamine side chain would be used as a means to differentiate the two 2-D depictions of 2,5-dimethoxy-4-x-phenethylamines from 3,6-dimethoxy-4-x-phenethylamines...
Otherwise one orientation of 2,5-dimethoxy-4-x-phenethylamine in 2-D would appear identical to 3,6-dimethoxy-4-x-phenethylamine, you can't have the same depiction represent two different molecules...
...so how to differentiate? The orientation of the double bonds!
Take 2,5-dimethoxyamphetamine in 3-D, when you rotate it, just as the methoxy groups appear to have changed orientation, so have the double bonds. This way you can tell an "upside down" or clockwise numbered 2,5-dimethoxy-4-x-phenethylamine from a "right side up" counterclockwise numbered 2,5-dimethoxy-4-x-phenethylamine, and they can both be distinguished from 3,5-dimethoxy-4-x-phenethylamines...
It's a simple solution, I can't believe I did not see it before, it correlates to the physical molecule, and clears up confusion regarding a single representation for two distinct compounds.
Please, just give what I'm saying a chance, and put it to the test.
Notice how shulgin, who depicts his phenethylamines clockwise, always has the double bonds oriented in a specific manner, and then when you look at David E. Nichols, who depicts his phenethylamines counter-clockwise, he also depicts his double bonds oriented opposite to shulgins!
Attachments
entheogenic-gnosis
Rising Star
Mindlusion, I am listening...
Please carefully read my posts, and examine the attached photos.
( I gave downwardfromzero a hard time over a misunderstanding in another thread, but I apologize for it, in that thread I really was not listening, well, we were both misunderstanding and I didn't help the situation, I'll put it that way, that was rude, and I apologize.
Listen very carefully...
Actually, here, do me a favor,
Depict in 2-D:
·2,5-dimethoxy-4-x-amphetamine (clockwise numbering and counter-clockwise numbering )
Then depict in 2-D :
·3,6-dimethoxy-4-x-amphetamine (clockwise numbering and counter-clockwise numbering)
And then post the 4 molecules next to each other.
-eg
Please carefully read my posts, and examine the attached photos.
( I gave downwardfromzero a hard time over a misunderstanding in another thread, but I apologize for it, in that thread I really was not listening, well, we were both misunderstanding and I didn't help the situation, I'll put it that way, that was rude, and I apologize.
Listen very carefully...
Actually, here, do me a favor,
Depict in 2-D:
·2,5-dimethoxy-4-x-amphetamine (clockwise numbering and counter-clockwise numbering )
Then depict in 2-D :
·3,6-dimethoxy-4-x-amphetamine (clockwise numbering and counter-clockwise numbering)
And then post the 4 molecules next to each other.
-eg
I drew some extra conformational changes to further illustrate my point...


entheogenic-gnosis
Rising Star
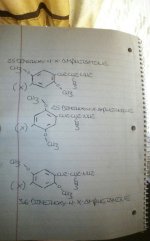
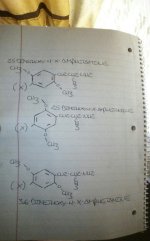
Or I'll put it this way:
Depict a 2-D representation of "molecule-A" as follows:
·Carbon 1 = ethylamine side chain
·Carbon 2 = methoxy group
·Carbon 3 = unsplit aromatic hydrogen atom
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = methoxy group
·Carbon 6 = unsplit aromatic hydrogen atom
Now, depict a 2-d representation of molecule-B as follows:
·Carbon 1 = ethylamine side chain
·Carbon 2 = unsplit aromatic hydrogen atom
·Carbon 3 = methoxy group
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = unsplit aromatic hydrogen atom
·Carbon 6 = methoxy group
-eg
Depict a 2-D representation of "molecule-A" as follows:
·Carbon 1 = ethylamine side chain
·Carbon 2 = methoxy group
·Carbon 3 = unsplit aromatic hydrogen atom
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = methoxy group
·Carbon 6 = unsplit aromatic hydrogen atom
Now, depict a 2-d representation of molecule-B as follows:
·Carbon 1 = ethylamine side chain
·Carbon 2 = unsplit aromatic hydrogen atom
·Carbon 3 = methoxy group
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = unsplit aromatic hydrogen atom
·Carbon 6 = methoxy group
-eg
entheogenic-gnosis
Rising Star
Mindlusion said:I drew some extra conformational changes to further illustrate my point...

Depict a 2-D 3,6-dimethoxy-4-x-amphetamine molecule.
-eg
entheogenic-gnosis said:Or I'll put it this way:
Depict a 2-D representation of "molecule-A" as follows:
·Carbon 1 = ethylamine side chain
·Carbon 2 = methoxy group
·Carbon 3 = unsplit aromatic hydrogen atom
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = methoxy group
·Carbon 6 = unsplit aromatic hydrogen atom
Now, depict a 2-d representation of molecule-B as follows:
·Carbon 1 = ethylamine side chain
·Carbon 2 = unsplit aromatic hydrogen atom
·Carbon 3 = methoxy group
·Carbon 4 = halogen or alkyl chain
·Carbon 5 = unsplit aromatic hydrogen atom
·Carbon 6 = methoxy group
-eg
These aren't a thing.
you can't just start making up your own things now
both molecules are identical, changing the numbering doesn't change anything, as i showed you 6 times in my image that i spent 5 minutes of my life putting together. like we've all said over and over. you number in the direction of the closest substituent relative to the ethylamine chain. In this case it is CARBON 2 that HAS METHOXY GROUP. SO DO IT
entheogenic-gnosis
Rising Star
So your saying it's not possible to place methoxy groupings to carbons 3 and 6 of a phenethylamine?
-eg
-eg
entheogenic-gnosis said:So your saying it's not possible to place methoxy groupings to carbons 3 and 6 of a phenethylamine?
-eg
4-bromophenethylamine is a symmetrical molecule across the 1,4 axis (disregarding chirality)
so that means the 3,6 position of the phenyl ring is the same as the 2,5 positions.
if you physically move the methoxy groups, one moves away from the chain towards the bromo, and one moves away from the bromo towards the chain. to give the same molecule.
the old terminology was ortho, meta para, for 1,2, 1,3 and 1,4.
say para-dimethoxy benzene. You could write this in 3 ways with numbers. 1,4 or 2,5, or 3,6. But since the molecule is completely symmetric, all three are the same. The is the case of 2c-b or DOB, when you are just alternating the two methoxy groups. 2,5 and 3,6 are the same due to symmetry. and 1,4 is taken by the bromo and ethylamine chain in this case.
Sorry for losing my patience a bit, im glad you take the time to learn about this stuff
entheogenic-gnosis
Rising Star
there are 6 available carbon atoms, all of which are just as viable for substitution as the last...
If what you ate saying is correct than how do you explain 2,5-dimethoxy-3,4-dimethyl-phenethylamine?
If 2 and 5 and 3 and 6 are the same atom, than how can position 3 have a methyl group attatched? Because if what your saying is correct it would already be filled by a methoxy group.
-eg
If what you ate saying is correct than how do you explain 2,5-dimethoxy-3,4-dimethyl-phenethylamine?
If 2 and 5 and 3 and 6 are the same atom, than how can position 3 have a methyl group attatched? Because if what your saying is correct it would already be filled by a methoxy group.
-eg
entheogenic-gnosis said:there are 6 available carbon atoms, all of which are just as viable for substitution as the last...
If what you ate saying is correct than how do you explain 2,5-dimethoxy-3,4-dimethyl-phenethylamine?
If 2 and 5 and 3 and 6 are the same atom, than how can position 3 have a methyl group attatched? Because if what your saying is correct it would already be filled by a methoxy group.
-eg
Think about it.
Ask yourself. Is this molecule symmetric in the same way as 4 bromo 2,5diMeO?
Don't memorize, apply the knowledge
entheogenic-gnosis
Rising Star
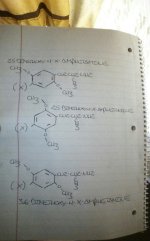
Put it to the test, in my drawing:
·the fisrt molecule depicts clockwise 2,5-dimethoxy-4-x-amphetamine, and the double bonds in the benzene ring reflect this.
· the second molecule depicts counter-clockwise 2,5-dimethoxy-4-x-phenethylamine and just as it appears that the methoxy orientations have switched, so to do the orientations of the double bonds, as you rotated the molecule on its axis.
·The third molecule depicts 3,6-dimethoxy-4-x-amphetamine, which can be distinguished from a clockwise 2,5-dimethoxy-4-x-amphetamine by the orientation of its double bonds
Take counter-clockwise numbered 2,5-dimethoxy-4-x-phenethylamine in 3-D, spin it on its axis until it appears that the methoxy substitutions have switched to clockwise orientation, keep your eye on the double bonds in the benzene ring as you rotate the molecule, the orientation of the double bonds in the benzene ring "switches" with the methoxy groupings.
-eg
·the fisrt molecule depicts clockwise 2,5-dimethoxy-4-x-amphetamine, and the double bonds in the benzene ring reflect this.
· the second molecule depicts counter-clockwise 2,5-dimethoxy-4-x-phenethylamine and just as it appears that the methoxy orientations have switched, so to do the orientations of the double bonds, as you rotated the molecule on its axis.
·The third molecule depicts 3,6-dimethoxy-4-x-amphetamine, which can be distinguished from a clockwise 2,5-dimethoxy-4-x-amphetamine by the orientation of its double bonds
Take counter-clockwise numbered 2,5-dimethoxy-4-x-phenethylamine in 3-D, spin it on its axis until it appears that the methoxy substitutions have switched to clockwise orientation, keep your eye on the double bonds in the benzene ring as you rotate the molecule, the orientation of the double bonds in the benzene ring "switches" with the methoxy groupings.
-eg