Loveall said:
I have found that longer basing gave higher yields when going from a few minutes to one hour. Will try a full day. The increase in yields was maybe 20% or so.
I wonder where is the upper limit of basing time for increasing yields? 1 hour, 24 hours, 1 week?
Is the salted #3 experiment crashing? The bottom of the paper strip is not very red (looks orange), you may need more citric acid for crashing to happen.
Run #3(A):
- 25% of the combined pulls;
- Salted with 2 mg citric per gram of solvent;
- Placed in the freezer;
It formed slushy ICE on top, no ICE on the bottom, no xtals, but it's still cloudy. This morning I poured it all into a strainer to separate out the slushy ICE. Then salted with an additional 1 mg citric per gram of solvent.
See photo of pH paper: far right is with additional 1 mg/gram, 2nd from right is immediately before adding the additional 1 mg/gram, 3rd from right is from last night so the color has faded.
Edit: Comparing to last night's photo, the green fades but maybe the red/orange does not fade. Also, 2nd from right may be more red than last night's 3rd from right because water ICE was removed before dipping the pH strip.
Now the solvent should have 3 mg citric per gram of solvent assuming proportional distribution of the original 2 mg citric in the liquid solvent versus the ICE which was separated out. Magnetic stir for 3 minutes, noticed powder falling to the bottom of the jar. Magnetic stir for another 5 minutes to see if the powder would dissolve. No, it all settled to the bottom of the jar (
see photo looking up at the bottom of the jar of the yellowish solvent, with the powder in the corner). Maybe it's mescaline citrate? Solvent is no longer cloudy. What's the best way to separate this powder from the solvent?
I separated the slushy top ICE into ethyl acetate (18.9 grams) and what appears to be water (2.7 grams). The water is evaporating into a small collection of xtals. The ethyl acetate looks to have nothing except greenish oils.
Loveall, have you tried pulling your xtalized ethyl acetate with water and then evaporating the water to see if you get additional yield?
Some changes happening with Run #2(A):
Jar was in the freezer and formed slushy ICE floating on top and ICE stuck to the bottom. I left it in the freezer and nothing else really happened. So last night I took the jar out (after 60 hours in the freezer) and I separated the top ICE, bottom ICE, and the liquid solvent (into a new, clean jar).
This morning I noticed the solvent jar had a small number of xtals at the bottom of the jar (
see photo of the jar with the dark emerald green solvent where there are a handful of xtals forming). I put that jar into the frig and a few more xtals formed along with a little glitter. Not much else after 4+ hours.
I evaporated the (now melted) bottom ICE and got xtals (
see photo of glass baking dish with water in the corner and lots of xtals forming) which I scraped into a powder (
photo shows slightly wet powder next to razor blade). The powder is off-white and tastes a little salty/bitter. Not sour. The top ICE evaporated to nothing. Just a small amount of greenish oil.
I'm debating what to do with this jar in the frig. It is still not quite clear. Wait longer, move to freezer? Add more citric? It currently has 1.375 mg citric per gram of solvent. I am currently salting new pulls at a minimum of 2 mg citric per gram of solvent and maybe 3 mg is better.
If you can get the cloudiness to crash, we will have process that was reproduced. Also, any results with malic?
It seems like we're starting to make progress with the citric. I haven't tested malic yet. The solubility of malic in ethyl acetate is supposedly much less than the solubility of citric. So I'm concerned we'll end up with excess malic in the end product.
I have about 480 grams of Run #3 combined pulls waiting to be salted. I figured I would split into 3 more samples of 160 grams each and try a few things:
(B): Salt with 3 mg citric per gram of solvent (maybe more?). Place in frig for a while to see if I get xtals before trying the freezer;
(C): Freeze
without adding citric. See if I get ICE. Then either let it melt back into the solvent or pull the ICE out before salting. The theory being excess water is interfering with xtal formation;
(D): Use citric acid monohydrate? This assumes Loveall might be using monohydrate rather than anhydrous. Still waiting for a definitive answer on that one. Loveall, can you order some anhydrous and test with that?
Thanks for the good work shroombee.
:thumb_up:










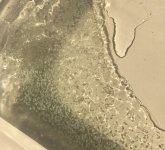

 . The picture with the yellow extract, right? I think you got it. I even see some smaller xtals sticking to the wall which is typical.
. The picture with the yellow extract, right? I think you got it. I even see some smaller xtals sticking to the wall which is typical.