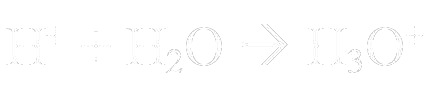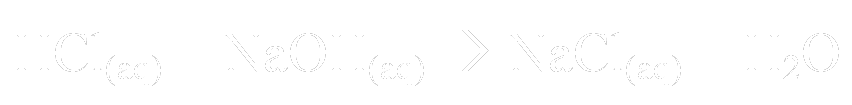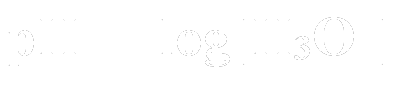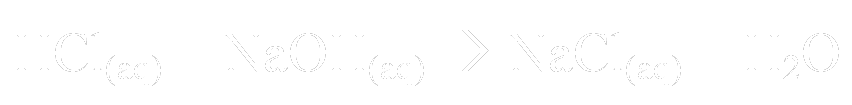This is a comprehensive guide on extracting DMT. This is not really a tek, it's more of a methodology. This guide goes through basic chemistry and how it relates to DMT extractions with respect to people with zero chemistry knowledge. It is pretty thorough, going through basic calculations and principles involved in the whole process.
"This guide is NOT designed for novices who take shortcuts and are impatient to try the spice. This is written with the intention that every reader will thoroughly read and reread in order to fully understand the theory of acid-base extractions."
This is a constant work in progress. If the almighty benzyme can review this, I will be eternally grateful.
Please share your comments, criticisms, suggestions, recommendations, insults, death threats etc. All are welcome. There may or may not be a 4th edition in the works, depending on the reception this one gets.
Purpose for this guide
This guide is designed for novices who aren’t familiar with chemistry. There are many novices who are very interested in DMT, but often get caught up in the scientific jargon. This, hopefully, will resolve that. This guide is NOT designed for novices who take shortcuts and are impatient to try the spice. This is written with the intention that every reader will thoroughly read and reread in order to fully understand the theory of acid-base extractions.
The secondary purpose for this guide is to provide a basic framework to conduct comparative assays on various plant material for comparison of active alkaloid levels.
NOTE: This guide isn’t meant to discourage novices from venturing into the world of extractions. The materials used here are all labware. Labware was used to maintain proper scientific procedures for the purpose of comparative analysis. Labware is not needed for extracting only. For guides on how to extract DMT with readily available materials, please consult MAX ION Tek or Earthwalkers ACRB Tek.
Which procedure should I use?
There are 2 main extraction techniques utilised in the extraction of DMT. The acid-to-base extraction (ATB) and the straight-to-base (STB) extraction. Both of these techniques rely on the precipitation of freebase DMT within a solvent. However, in order to obtain freebase DMT, it must be extracted from the plant material. DMT is soluble in acids, and so ATB is widely used. ATB is preferable to STB for only one circumstance; the species of plant material used. Typically, ATB is preferred when using fat laden plant species, such as Acacias. The DMT is located in the central vacuole of the plant cell, so in chemical processing, we try to break apart all the plant structures. This is the reason for acid simmering steps that last several hours. However, when you break apart the entire structure, it is inevitable that you will extract some unwanted compounds, most common being fats and oils. These are harmless, but lower the purity of the final product. In DMT extractions, we try to extract alkaloids only, and leaving behind all the plant fats and oils. It is impossible to know the constituents of your product by the naked eye, but all successful extractions have similar properties. In order to fully analyse your handiwork, it is recommended to run a thin-layer chromatography (TLC) on the sample. The DMT Nexus has recently organised TLC kits for the purpose of extraction analyses, which can be bought on the link below:
It is not necessary to analyse the product if it looks similar to other extracts, but it’s a technique that you have at your disposal should you wish to pursue an active interest in the extraction techniques of plant-based entheogens.
STB is typically used for not-so-fat-laden species, such as Mimosa hostilis. The procedure is exactly the same as ATB, without the acid.
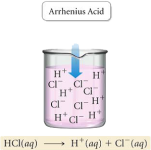
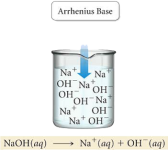
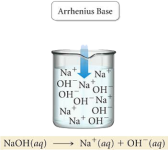
It would be career suicide for a chemistry lecturer to talk about acids and bases without mentioning Brønsted and Lowry. Brønsted and Lowry were two chemists who independently defined what an acid and a base is. But before we go into the Brønsted-Lowry definition, it’s better to understand the history of defining acids and bases. So first, we’ll discuss the Arrhenius definition. Svante Arrhenius was a Swedish chemist, who in the 1880’s described an acid as “A substance that produces H+ ions in aqueous solution”, and a base as “A substance that produces OH- in aqueous solution”.
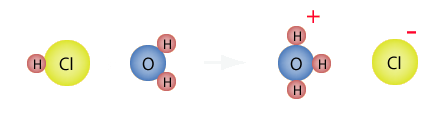
A practical explanation of this is HCl(g). Notice the subscript with a “g”? That means the compound is in a gaseous state. HCl(aq) is hydrochloric acid and HCl(g) is hydrogen chloride. In gaseous form HCl, is a covalent compound, i.e. it is not bound to anything else except itself. Once it enters the aqueous phase by being dissolved in water, it forms ionic bonds with water molecules. Therefore, hydrochloric acid is an ionic solution. However, when we say that “it” forms ionic bonds with water, we refer to the H+ ion only. Cl- does not play a part in acids or bases.
H+ binds to water to form an ionic compound called a hydronium ion. The chemical equation looks like this:
Chemists often use H+ and H3O+ interchangeably. They both denote the same thing, which is an H+ that has been dissolved in water.
A little factoid here; an H+ ion is just a proton. A hydrogen atom consists of 1 proton in the nucleus and 1 electron spinning around it. Once the atom loses the electron and becomes H+, it is left with only a proton.
So with this in mind, we’ll now move on to the currently accepted definition of acids and bases, as proposed by Brønsted and Lowry. The Arrhenius definition isn’t wrong, per se, it’s just that the Brønsted-Lowry definition is more correct, if that makes sense.
The Brønsted-Lowry definition is focused on the transfer of H+ ions rather than producing them. Since a H+ ion is a proton, Brønsted-Lowry defined an acid and base as this:
Acid: A proton donor
Base: A proton acceptor
You may ask, “how are the two definitions different?” For the purposes of DMT extraction, both are correct. But for advanced chemistry, the Arrhenius definition becomes a bit confusing, mainly because ammonia (NH3) acts as a base. This is why the Arrhenius definition doesn’t hold up to the Brønsted-Lowry definition. We won’t go into that, but if you’re interested, check out the acid-base section of any chemistry textbook.
So, what happens in an acid-base extraction? The best way to explain this is through a chemical equation.
The general rule is: acid + base --> salt + water
So if we use hydrochloric acid and sodium hydroxide, the equation is this:
So the products of this reaction are sodium chloride (table salt) and water. This only happens when equal concentrations of HCl and NaOH are mixed. If the HCl is in excess, for example, all of the NaOH will be consumed to make NaCl(aq), but there will still be some HCl in the solution. Therefore, the solution will still be acidic, albeit not as acidic.
So now we’ve established what an acid is, but haven’t fully gone into what a base is. I’ve left it for this section since it is useful for explaining pH as well.
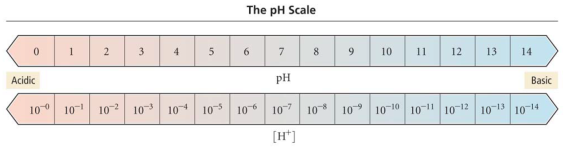
pH stands for “potential hydrogen”. On the pH scale, 7 is neutral. Lower than 7 is acidic, higher than 7 is basic or alkaline.
The calculation of pH is:
The pH scale is a logarithmic scale.
An increase of 1 on the pH scale corresponds to a decrease in the concentration of H3O+ by a factor of 10.
Molarity is the concentration of a solution. If we say 2M HCl, that means the HCl has a concentration of 2 moles per litre. So, what is a mole? It is a small furry animal that likes to dig. It is also an SI unit of measurement that indicates amount of a substance. Moles are dependent on two things, the mass of the substance, and the molecular mass of the substance. What’s molecular mass? To understand this, we need to break down the Periodic Table of Elements.
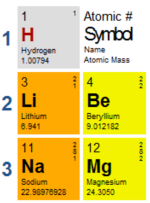
Let’s zoom in to the first element, hydrogen.
The atomic number is used to order all the elements according to their atomic size. The symbol and name is self-explanatory. What we’re interested in is the atomic mass. Atomic mass is the same as molecular mass, which is also the same as molar mass. It is the mass of one mole of that element, in grams. So 1 mole of lithium is 6.941 grams.
Here is the calculation for number of moles:
So if you have 3 grams of lithium, the calculation is:
Number of moles = 3 / 6.941
= 0.43 mol
To calculate molarity, the formula is:
So let’s use a real life example pertaining to DMT to sum this all up. We want to make a 2M solution of NaOH.
First thing is to weigh out 2 moles of NaOH. Using the Periodic Table, 1 mole of NaOH is:
22.99 (molar mass of Na) + 15.99 (molar mass of O) + 1.008 (molar mass of H) = 39.99 g.
Since we want 2 moles, we double this amount. So 79.98 g. We then prepare 500ml of COLD distilled water. We slowly dissolve all of the NaOH into the water. Once it is fully dissolved, we check the volume again. It should be more than 500ml. We then bring up the volume to 1L with fresh distilled water (which does not mean add another 500ml). And there we go, we have just made a 2M NaOH solution.
It is important not to just add all of the NaOH into 1L of water. Just because it will dissolve doesn’t mean it won’t occupy space. If you added all of the NaOH into 1L of water, the final volume will be much higher than 1L.
Polarity refers to the overall charge of the molecule. Polar means it is slightly charged, non-polar means it is not charged at all.
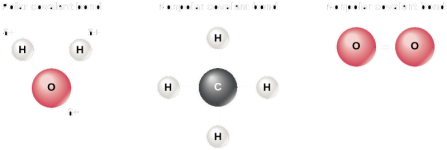
Water has an overall positive charge, it is polar. The charges on methane cancel out, so it is non-polar. Oxygen is diatomic, and only has one kind of atom, so it is non-polar.
This is essentially all there is to polarity in relation to extractions. The golden rule of polarity is:
Polar things will dissolve in other polar things. Non-polar things will dissolve in other non-polar things. Polar things will NOT dissolve in non-polar things. Non-polar things will NOT dissolve in polar things. I know, very scientific to use the word “things”.
There is A LOT more to polarity than what is said here, but this information should be enough for extractions. Again, if you’re interested in chemistry, pick up any chemistry textbook.
When DMT is dissolved in hydrochloric acid (HCl), it forms DMT hydrochloride, when it is dissolved in acetic acid (CH3COOH), it forms DMT acetate. From here, the acid can then be neutralised with a base. In chemical terms:
So hydrochloric acid and sodium hydroxide (NaOH) will look like this:
(If you recall, the subscript denotes the state the compound is in. Aq means aqueous. HCl is actually a gas and only becomes an acid when it is dissolved in water). NaCl is table salt. Once the acid has been neutralised, which includes the DMT hydrochloride, the DMT has nowhere else to go, and is floating around in the solution in freebase form. From here, a solvent is added to allow the DMT to migrate to a liquid that has a much lower freezing point than what conventional freezers can produce. This allows for the use of conventional household freezers to freeze precipitate the DMT within the solvent. This has 2 advantages over precipitation by evaporation. Firstly, it takes a much shorter amount of time. The time for a freeze precipitation is in the span of a day or two, but evaporation will take a few days to a few weeks to fully evaporate, depending on your climate. The second advantage is that you will be keeping the solvent. The solvent can be washed and used ad infinitum. Having to buy new solvent for every extract will end up costing a lot, if you have many failures or conduct many extracts.
3. Extraction Methodology
MATERIALS:
1) 100g of your plant material of choice. ACRB (Acacia confusa root bark) powder was used here.
2) Magnetic hotplate stirrer and stir bar
3) A strong magnet (to remove the stir bar)
4) Pyrex glass water bath
5) 1 x 1000ml Erlenmeyer flask
6) 1x ≥ 1000ml beaker
7) 500 mL measuring cylinder
8 ) 50 mL turkey baster
9) 1.0 ml eyedropper
10) Plastic funnel
11) Glass thermometer
12) 0.001M HCl stock solution (see first step in this section on how to prepare stock solution)
13) 5M NaOH stock solution (see first step in this section on how to prepare stock solution)
14) Naphtha or any non-polar solvent.
15) 80g of non-iodised NaCl (table salt).
16) Rectangular Pyrex dish (preferably with an airtight and watertight locking lid)
17) Razor blade
METHOD:
Preparing stock solution:
NOTE: The HCl and NaOH were acquired from a hardware store. According to their packaging, the HCl states “Contains min. 320g/L of hydrochloric acid solution”. For NaOH, the packaging states “Contains: 98% Sodium hydroxide.”
1) Acquire 2 large and fairly sturdy HDPE containers. Distilled water containers found at hardware stores are suitable, but something a bit sturdier would be preferred. Pick a size appropriate for you. Recommended minimum is 5L capacity.
2) Thoroughly wash the containers. This is not necessary if the container was for distilled water. It is ideal to use the distilled water to make the stock solution in the first place.
3) Calculate the amounts required to get achieve desired concentration.
FOR HCl:
Assuming the concentration is 320g/L. A pH of 2-4 is ideal. For arguments sake, we will calculate a pH of 3. To achieve this pH we will use the following formula.
As can be seen on the scale in the pH section, the concentration is 10-3. This is equates to 0.001M HCl, which is why this particular concentration is specified in the Materials section. Now, we need to calculate the concentration of the undiluted HCl.
First, we calculate the number of moles:
Number of moles = Mass (in grams) / molecular mass
= 320 / 36.458 (atomic weight of H and Cl)
= 8.777…
Therefore, the number of moles in 1L is 8.777. Which means the molarity is 8.78M (rounded to 2 significant figures).
Now, we figure out how much we need to dilute the concentrated HCl solution.
Let’s say we want 10L of final stock solution.
We use the following formula (which we haven’t covered before):
1 refers to the concentrated solution. 2 refers to the diluted solution. C is concentration and V is volume. For volume, it’s best to use mL.
So we have the following information: C1 = 8.77, C2 = 0.001, V2 = 10000 (10L). We want V1, which will tell us the volume of concentrated HCl to add into 10L of water.
8.77 x V1 = 0.001 x 10000
V1 = (0.001 x 10000) / 8.77
= 1.14 mL
Therefore, we add 1.14 mL into 10L of water. If you want to be ultra-scientific, you would add 1.14 mL of acid into 9.99886 L of water, since the concentrated solution is contributing to the overall volume. For our purposes, this isn’t necessary.
FOR NaOH:
Since NaOH is in a solid state, we will measure out the amount of NaOH needed. First we need to calculate the mass of 1 mole of NaOH.
1 mole of NaOH is:
22.99 (molar mass of Na) + 15.99 (molar mass of O) + 1.008 (molar mass of H) = 39.99 g.
We want 5 M, so we multiply this mass by 5, which gives us 199.95 grams per litre of water.
Preparing NaOH stock solution is a bit different to HCl, due to safety concerns. Pouring the NaOH granules into water will create an exothermic reaction, i.e. it releases heat. A LOT of it. So we cannot prepare the entire 10L in one go.
We must create the solution 1 litre at a time.
1) Prepare 500ml of COLD distilled water in a 1L borosilicate Erlenmeyer flask.
2) Slowly dissolve all of the NaOH into the water. It is advisable to add a small amount, and swirl the solution around to dissolve it. Then add more and swirl it again. Repeat until all NaOH is dissolved in solution. This will prevent a rapid increase in temperature.
3) Bring up the volume to 1L with fresh distilled water (which does not mean add another 500ml). An Erlenmeyer flask is not known for its accuracy in measuring volume, but for our intents and purposes, it will suffice.
4) Leave the flask to cool down. Pouring hot NaOH solution into a HDPE container may damage the container, so it’s best to err on the safe side.
5) Repeat steps 1-4 up till the desired volume is achieved.
This is a tedious process, but it is necessary. Given the quantities that are being used per extraction, this stock solution will last you a lifetime. Literally.
It is not required to be ultra-precise with preparing any solution for a DMT extract, but is recommended.
1) Place Pyrex glass water bath on magnetic hotplate stirrer.
2) Pour 600mL of 0.001M HCl stock solution into a 1000mL Erlenmeyer flask.
3) Drop the stir bar into the Erlenmeyer flask, by tilting the flask and allowing the stir bar to roll down the side. Do not drop it straight in.
4) Place Erlenmeyer flask in water bath. Fill the water bath with warm water until it is level with the solution in the flask.
5) Turn the heat and stir function on. Do not set either too high. Stir bar should be swirling the solution gently. The ideal temperature for the acid solution in this extraction is 60°C.
6) Slowly add 100g of ACRB powder into the flask using the plastic funnel. Try to prevent the powder from touching the sides of the flask. NOTE: It is likely that the stir bar will cease to spin when adding either too much powder or adding it too quick. In the event this happens, occasional manual swirling is required.
7) Leave the flask in the water bath for a minimum of 2 hours. If the stir bar ceases to spin, it is advisable to remove it now using the strong magnet. Simply place the magnet on the outside surface of the flask. Move the magnet around if it is not attracting the stir bar. Once it is attracted, drag the magnet along the surface of the flask and bring it up to the mouth of the flask. Once it is near the mouth, place the flask on a flat surface and use a gloved hand or tweezers to pick up the stir bar. Moving the magnet too rapidly can cause the stir bar to fall back down into the solution. This may cause damage to your flask. Finesse is key here.
8 ) After 2 hours, add 80g of non-iodised NaCl (table salt)
9) Slowly pour 100mL of 5M NaOH stock solution into the flask. The solution should go black. Turn the heat down a little bit. Ideal temperature is 50-60°C.
10) Leave the flask in the water bath for at least 1 hour. A longer wait time can be beneficial. Swirl occasionally if the stir bar ceases to move or was removed in step 7.
11) Remove stir bar if it hasn’t been removed already.
12) Add 80mL of naphtha or your non-polar solvent into the flask.
13) Pour entire contents of flask into the ≥ 1000ml beaker. Pour back and forth between the flask and beaker. This will thoroughly mix the 2 layers. The more you mix the layers, the more surface of the solvent will be exposed to the freebase DMT. Alternatively, if you have an alkali-resistant stopper, you can use it on the flask and shake. Do not shake too vigourously. There will be pressure buildup. Remember to remove the stopper after shaking to prevent pressure buildup.
14) Pour all the solution into the flask and let it sit in the water bath for 10 minutes or until both layers are clearly formed.
15) Careful decant this solution from the flask into the 500mL measuring cylinder. Since the non-polar solvent layer is on top, the majority of it will be poured into the cylinder. NOTE: Do not pour all of the solution in; the cylinder cannot hold it all. Pour as much as the cylinder can hold, giving some leeway to prevent spills.
16) Using a turkey baster, carefully pipette the top non-polar solvent into a rectangular Pyrex dish. Use two hands to keep the turkey baster steady, and pipette by touching the tip of the turkey baster to the side of the cylinder, just below the surface of the non-polar solvent. Stop when you’re getting too close to the basified layer.
17) Using the 1.0mL eyedropper, the remainder of the non-polar solvent can be pipetted. You can rest the palm of your pipetting hand against the side of the cylinder to keep it steady. Your other hand should hold the cylinder to prevent it from falling. The tip of the eyedropper should be resting against the inner surface of the cylinder and just under the surface of the solvent layer.
18 ) Repeat steps 12-17 thrice.
19) Place the rectangular Pyrex dish with the solvent into the freezer. Make sure the lid is locked in. If your dish does not come with a locking lid, then wrap the opening of the dish with cling film and place the entire dish into a large zip lock bag. Zip it up, and place in the freezer. Alternatively, you can let it evaporate completely in a dry place. If evaporating, skip to step 22.
20) Leave the dish in the freezer for a minimum of 24 hours. Do not disturb the dish before 24 hours are up, the crystals may not form properly otherwise.
21) After 24 hours, carefully decant the solvent into another container. It is recommended that you keep the solvent in another glass container for long term storage. This used solvent can be used ad infinitum.
22) Allow the dish to dry, either by focusing a fan into the interior, or letting it evaporate.
23) Scrape up the precipitate with a razor blade, and place on a small section of baking paper. This can be wrapped up, and stored in a cool, dark and dry place.
So there you have it. You have successfully extracted N,N-Dimethyltryptamine. What you do with this sacred molecule is up to you. “With great power comes great responsibility” - Voltaire.
I would like to give thanks to all the people on the DMT-Nexus who have provided valuable information and support in regards to everything DMT related, and life in general.
A special thanks go out to cyb, chemisTryptaMan and Earthwalker who provided the basic structure of this methodology. All I did was tweak their work. This would not be possible without them. Thanks to some one (that's the username: some one) for pointing out some errors. Also a special shout out to resident chemical supergenius, benzyme.
cyb, chemisTryptaMan (2013). The cyb/CTM ‘Maximum Yield’ ATB Salt tek MAX ION. Wherever the hell cyb and CTM are from: DMT-Nexus
Earthwalker (2014). ACRB TEK 100g "PICS" (Newbie Friendly). Wherever the hell EW is from: DMT-Nexus
Tro, N. (2011). Chemistry. 2nd ed. Upper Saddle River, New Jersey: Pearson.
Various pictures taken from this textbook and stolen from Google Image searches. Sorry.
"This guide is NOT designed for novices who take shortcuts and are impatient to try the spice. This is written with the intention that every reader will thoroughly read and reread in order to fully understand the theory of acid-base extractions."
This is a constant work in progress. If the almighty benzyme can review this, I will be eternally grateful.
Please share your comments, criticisms, suggestions, recommendations, insults, death threats etc. All are welcome. There may or may not be a 4th edition in the works, depending on the reception this one gets.
NOW WITH THE BENZYME SEAL OF APPROVAL!

TABLE OF CONTENTS
1. Introduction
2. Chemistry 101
2.1. The Theory of Acid-Base Reactions
2.2. The Theory of pH
2.3. Molarity
2.4. What is Polarity?
2.5. So what does this have to do with DMT?
3. Extraction Methodology
4. Afterword
5. References
1. Introduction:

TABLE OF CONTENTS
1. Introduction
2. Chemistry 101
2.1. The Theory of Acid-Base Reactions
2.2. The Theory of pH
2.3. Molarity
2.4. What is Polarity?
2.5. So what does this have to do with DMT?
3. Extraction Methodology
4. Afterword
5. References
1. Introduction:
Purpose for this guide
This guide is designed for novices who aren’t familiar with chemistry. There are many novices who are very interested in DMT, but often get caught up in the scientific jargon. This, hopefully, will resolve that. This guide is NOT designed for novices who take shortcuts and are impatient to try the spice. This is written with the intention that every reader will thoroughly read and reread in order to fully understand the theory of acid-base extractions.
The secondary purpose for this guide is to provide a basic framework to conduct comparative assays on various plant material for comparison of active alkaloid levels.
NOTE: This guide isn’t meant to discourage novices from venturing into the world of extractions. The materials used here are all labware. Labware was used to maintain proper scientific procedures for the purpose of comparative analysis. Labware is not needed for extracting only. For guides on how to extract DMT with readily available materials, please consult MAX ION Tek or Earthwalkers ACRB Tek.
Which procedure should I use?
There are 2 main extraction techniques utilised in the extraction of DMT. The acid-to-base extraction (ATB) and the straight-to-base (STB) extraction. Both of these techniques rely on the precipitation of freebase DMT within a solvent. However, in order to obtain freebase DMT, it must be extracted from the plant material. DMT is soluble in acids, and so ATB is widely used. ATB is preferable to STB for only one circumstance; the species of plant material used. Typically, ATB is preferred when using fat laden plant species, such as Acacias. The DMT is located in the central vacuole of the plant cell, so in chemical processing, we try to break apart all the plant structures. This is the reason for acid simmering steps that last several hours. However, when you break apart the entire structure, it is inevitable that you will extract some unwanted compounds, most common being fats and oils. These are harmless, but lower the purity of the final product. In DMT extractions, we try to extract alkaloids only, and leaving behind all the plant fats and oils. It is impossible to know the constituents of your product by the naked eye, but all successful extractions have similar properties. In order to fully analyse your handiwork, it is recommended to run a thin-layer chromatography (TLC) on the sample. The DMT Nexus has recently organised TLC kits for the purpose of extraction analyses, which can be bought on the link below:
It is not necessary to analyse the product if it looks similar to other extracts, but it’s a technique that you have at your disposal should you wish to pursue an active interest in the extraction techniques of plant-based entheogens.
STB is typically used for not-so-fat-laden species, such as Mimosa hostilis. The procedure is exactly the same as ATB, without the acid.
2. Chemistry 101:
2.1 The theory of acid-base reactions
2.1 The theory of acid-base reactions
It would be career suicide for a chemistry lecturer to talk about acids and bases without mentioning Brønsted and Lowry. Brønsted and Lowry were two chemists who independently defined what an acid and a base is. But before we go into the Brønsted-Lowry definition, it’s better to understand the history of defining acids and bases. So first, we’ll discuss the Arrhenius definition. Svante Arrhenius was a Swedish chemist, who in the 1880’s described an acid as “A substance that produces H+ ions in aqueous solution”, and a base as “A substance that produces OH- in aqueous solution”.
A practical explanation of this is HCl(g). Notice the subscript with a “g”? That means the compound is in a gaseous state. HCl(aq) is hydrochloric acid and HCl(g) is hydrogen chloride. In gaseous form HCl, is a covalent compound, i.e. it is not bound to anything else except itself. Once it enters the aqueous phase by being dissolved in water, it forms ionic bonds with water molecules. Therefore, hydrochloric acid is an ionic solution. However, when we say that “it” forms ionic bonds with water, we refer to the H+ ion only. Cl- does not play a part in acids or bases.
H+ binds to water to form an ionic compound called a hydronium ion. The chemical equation looks like this:
Chemists often use H+ and H3O+ interchangeably. They both denote the same thing, which is an H+ that has been dissolved in water.
A little factoid here; an H+ ion is just a proton. A hydrogen atom consists of 1 proton in the nucleus and 1 electron spinning around it. Once the atom loses the electron and becomes H+, it is left with only a proton.
So with this in mind, we’ll now move on to the currently accepted definition of acids and bases, as proposed by Brønsted and Lowry. The Arrhenius definition isn’t wrong, per se, it’s just that the Brønsted-Lowry definition is more correct, if that makes sense.
The Brønsted-Lowry definition is focused on the transfer of H+ ions rather than producing them. Since a H+ ion is a proton, Brønsted-Lowry defined an acid and base as this:
Acid: A proton donor
Base: A proton acceptor
You may ask, “how are the two definitions different?” For the purposes of DMT extraction, both are correct. But for advanced chemistry, the Arrhenius definition becomes a bit confusing, mainly because ammonia (NH3) acts as a base. This is why the Arrhenius definition doesn’t hold up to the Brønsted-Lowry definition. We won’t go into that, but if you’re interested, check out the acid-base section of any chemistry textbook.
So, what happens in an acid-base extraction? The best way to explain this is through a chemical equation.
The general rule is: acid + base --> salt + water
So if we use hydrochloric acid and sodium hydroxide, the equation is this:
So the products of this reaction are sodium chloride (table salt) and water. This only happens when equal concentrations of HCl and NaOH are mixed. If the HCl is in excess, for example, all of the NaOH will be consumed to make NaCl(aq), but there will still be some HCl in the solution. Therefore, the solution will still be acidic, albeit not as acidic.
2.2 The theory of pH
So now we’ve established what an acid is, but haven’t fully gone into what a base is. I’ve left it for this section since it is useful for explaining pH as well.
pH stands for “potential hydrogen”. On the pH scale, 7 is neutral. Lower than 7 is acidic, higher than 7 is basic or alkaline.
The calculation of pH is:
The pH scale is a logarithmic scale.
An increase of 1 on the pH scale corresponds to a decrease in the concentration of H3O+ by a factor of 10.
2.3 Molarity
Molarity is the concentration of a solution. If we say 2M HCl, that means the HCl has a concentration of 2 moles per litre. So, what is a mole? It is a small furry animal that likes to dig. It is also an SI unit of measurement that indicates amount of a substance. Moles are dependent on two things, the mass of the substance, and the molecular mass of the substance. What’s molecular mass? To understand this, we need to break down the Periodic Table of Elements.
Let’s zoom in to the first element, hydrogen.
The atomic number is used to order all the elements according to their atomic size. The symbol and name is self-explanatory. What we’re interested in is the atomic mass. Atomic mass is the same as molecular mass, which is also the same as molar mass. It is the mass of one mole of that element, in grams. So 1 mole of lithium is 6.941 grams.
Here is the calculation for number of moles:
Number of moles = Mass (in grams) / molecular mass
So if you have 3 grams of lithium, the calculation is:
Number of moles = 3 / 6.941
= 0.43 mol
To calculate molarity, the formula is:
Number of moles = concentration x volume (in litres)
OR
Concentration = number of moles / volume (in litres)
OR
Concentration = number of moles / volume (in litres)
So let’s use a real life example pertaining to DMT to sum this all up. We want to make a 2M solution of NaOH.
First thing is to weigh out 2 moles of NaOH. Using the Periodic Table, 1 mole of NaOH is:
22.99 (molar mass of Na) + 15.99 (molar mass of O) + 1.008 (molar mass of H) = 39.99 g.
Since we want 2 moles, we double this amount. So 79.98 g. We then prepare 500ml of COLD distilled water. We slowly dissolve all of the NaOH into the water. Once it is fully dissolved, we check the volume again. It should be more than 500ml. We then bring up the volume to 1L with fresh distilled water (which does not mean add another 500ml). And there we go, we have just made a 2M NaOH solution.
It is important not to just add all of the NaOH into 1L of water. Just because it will dissolve doesn’t mean it won’t occupy space. If you added all of the NaOH into 1L of water, the final volume will be much higher than 1L.
2.4 What is polarity?
Polarity refers to the overall charge of the molecule. Polar means it is slightly charged, non-polar means it is not charged at all.

Water has an overall positive charge, it is polar. The charges on methane cancel out, so it is non-polar. Oxygen is diatomic, and only has one kind of atom, so it is non-polar.
This is essentially all there is to polarity in relation to extractions. The golden rule of polarity is:
Like dissolves like
Polar things will dissolve in other polar things. Non-polar things will dissolve in other non-polar things. Polar things will NOT dissolve in non-polar things. Non-polar things will NOT dissolve in polar things. I know, very scientific to use the word “things”.
There is A LOT more to polarity than what is said here, but this information should be enough for extractions. Again, if you’re interested in chemistry, pick up any chemistry textbook.
2.5 So what does this have to do with DMT?
When DMT is dissolved in hydrochloric acid (HCl), it forms DMT hydrochloride, when it is dissolved in acetic acid (CH3COOH), it forms DMT acetate. From here, the acid can then be neutralised with a base. In chemical terms:
acid + base --> salt + water
So hydrochloric acid and sodium hydroxide (NaOH) will look like this:
(If you recall, the subscript denotes the state the compound is in. Aq means aqueous. HCl is actually a gas and only becomes an acid when it is dissolved in water). NaCl is table salt. Once the acid has been neutralised, which includes the DMT hydrochloride, the DMT has nowhere else to go, and is floating around in the solution in freebase form. From here, a solvent is added to allow the DMT to migrate to a liquid that has a much lower freezing point than what conventional freezers can produce. This allows for the use of conventional household freezers to freeze precipitate the DMT within the solvent. This has 2 advantages over precipitation by evaporation. Firstly, it takes a much shorter amount of time. The time for a freeze precipitation is in the span of a day or two, but evaporation will take a few days to a few weeks to fully evaporate, depending on your climate. The second advantage is that you will be keeping the solvent. The solvent can be washed and used ad infinitum. Having to buy new solvent for every extract will end up costing a lot, if you have many failures or conduct many extracts.
3. Extraction Methodology
MATERIALS:
1) 100g of your plant material of choice. ACRB (Acacia confusa root bark) powder was used here.
2) Magnetic hotplate stirrer and stir bar
3) A strong magnet (to remove the stir bar)
4) Pyrex glass water bath
5) 1 x 1000ml Erlenmeyer flask
6) 1x ≥ 1000ml beaker
7) 500 mL measuring cylinder
8 ) 50 mL turkey baster
9) 1.0 ml eyedropper
10) Plastic funnel
11) Glass thermometer
12) 0.001M HCl stock solution (see first step in this section on how to prepare stock solution)
13) 5M NaOH stock solution (see first step in this section on how to prepare stock solution)
14) Naphtha or any non-polar solvent.
15) 80g of non-iodised NaCl (table salt).
16) Rectangular Pyrex dish (preferably with an airtight and watertight locking lid)
17) Razor blade
METHOD:
Preparing stock solution:
NOTE: The HCl and NaOH were acquired from a hardware store. According to their packaging, the HCl states “Contains min. 320g/L of hydrochloric acid solution”. For NaOH, the packaging states “Contains: 98% Sodium hydroxide.”
1) Acquire 2 large and fairly sturdy HDPE containers. Distilled water containers found at hardware stores are suitable, but something a bit sturdier would be preferred. Pick a size appropriate for you. Recommended minimum is 5L capacity.
2) Thoroughly wash the containers. This is not necessary if the container was for distilled water. It is ideal to use the distilled water to make the stock solution in the first place.
3) Calculate the amounts required to get achieve desired concentration.
FOR HCl:
Assuming the concentration is 320g/L. A pH of 2-4 is ideal. For arguments sake, we will calculate a pH of 3. To achieve this pH we will use the following formula.
As can be seen on the scale in the pH section, the concentration is 10-3. This is equates to 0.001M HCl, which is why this particular concentration is specified in the Materials section. Now, we need to calculate the concentration of the undiluted HCl.
First, we calculate the number of moles:
Number of moles = Mass (in grams) / molecular mass
= 320 / 36.458 (atomic weight of H and Cl)
= 8.777…
Therefore, the number of moles in 1L is 8.777. Which means the molarity is 8.78M (rounded to 2 significant figures).
Now, we figure out how much we need to dilute the concentrated HCl solution.
Let’s say we want 10L of final stock solution.
We use the following formula (which we haven’t covered before):
C1V1 = C2V2
1 refers to the concentrated solution. 2 refers to the diluted solution. C is concentration and V is volume. For volume, it’s best to use mL.
So we have the following information: C1 = 8.77, C2 = 0.001, V2 = 10000 (10L). We want V1, which will tell us the volume of concentrated HCl to add into 10L of water.
8.77 x V1 = 0.001 x 10000
V1 = (0.001 x 10000) / 8.77
= 1.14 mL
Therefore, we add 1.14 mL into 10L of water. If you want to be ultra-scientific, you would add 1.14 mL of acid into 9.99886 L of water, since the concentrated solution is contributing to the overall volume. For our purposes, this isn’t necessary.
FOR NaOH:
Since NaOH is in a solid state, we will measure out the amount of NaOH needed. First we need to calculate the mass of 1 mole of NaOH.
1 mole of NaOH is:
22.99 (molar mass of Na) + 15.99 (molar mass of O) + 1.008 (molar mass of H) = 39.99 g.
We want 5 M, so we multiply this mass by 5, which gives us 199.95 grams per litre of water.
Preparing NaOH stock solution is a bit different to HCl, due to safety concerns. Pouring the NaOH granules into water will create an exothermic reaction, i.e. it releases heat. A LOT of it. So we cannot prepare the entire 10L in one go.
We must create the solution 1 litre at a time.
1) Prepare 500ml of COLD distilled water in a 1L borosilicate Erlenmeyer flask.
2) Slowly dissolve all of the NaOH into the water. It is advisable to add a small amount, and swirl the solution around to dissolve it. Then add more and swirl it again. Repeat until all NaOH is dissolved in solution. This will prevent a rapid increase in temperature.
3) Bring up the volume to 1L with fresh distilled water (which does not mean add another 500ml). An Erlenmeyer flask is not known for its accuracy in measuring volume, but for our intents and purposes, it will suffice.
4) Leave the flask to cool down. Pouring hot NaOH solution into a HDPE container may damage the container, so it’s best to err on the safe side.
5) Repeat steps 1-4 up till the desired volume is achieved.
This is a tedious process, but it is necessary. Given the quantities that are being used per extraction, this stock solution will last you a lifetime. Literally.
It is not required to be ultra-precise with preparing any solution for a DMT extract, but is recommended.
Acid-base extraction:
1) Place Pyrex glass water bath on magnetic hotplate stirrer.
2) Pour 600mL of 0.001M HCl stock solution into a 1000mL Erlenmeyer flask.
3) Drop the stir bar into the Erlenmeyer flask, by tilting the flask and allowing the stir bar to roll down the side. Do not drop it straight in.
4) Place Erlenmeyer flask in water bath. Fill the water bath with warm water until it is level with the solution in the flask.
5) Turn the heat and stir function on. Do not set either too high. Stir bar should be swirling the solution gently. The ideal temperature for the acid solution in this extraction is 60°C.
6) Slowly add 100g of ACRB powder into the flask using the plastic funnel. Try to prevent the powder from touching the sides of the flask. NOTE: It is likely that the stir bar will cease to spin when adding either too much powder or adding it too quick. In the event this happens, occasional manual swirling is required.
7) Leave the flask in the water bath for a minimum of 2 hours. If the stir bar ceases to spin, it is advisable to remove it now using the strong magnet. Simply place the magnet on the outside surface of the flask. Move the magnet around if it is not attracting the stir bar. Once it is attracted, drag the magnet along the surface of the flask and bring it up to the mouth of the flask. Once it is near the mouth, place the flask on a flat surface and use a gloved hand or tweezers to pick up the stir bar. Moving the magnet too rapidly can cause the stir bar to fall back down into the solution. This may cause damage to your flask. Finesse is key here.
8 ) After 2 hours, add 80g of non-iodised NaCl (table salt)
9) Slowly pour 100mL of 5M NaOH stock solution into the flask. The solution should go black. Turn the heat down a little bit. Ideal temperature is 50-60°C.
10) Leave the flask in the water bath for at least 1 hour. A longer wait time can be beneficial. Swirl occasionally if the stir bar ceases to move or was removed in step 7.
11) Remove stir bar if it hasn’t been removed already.
12) Add 80mL of naphtha or your non-polar solvent into the flask.
13) Pour entire contents of flask into the ≥ 1000ml beaker. Pour back and forth between the flask and beaker. This will thoroughly mix the 2 layers. The more you mix the layers, the more surface of the solvent will be exposed to the freebase DMT. Alternatively, if you have an alkali-resistant stopper, you can use it on the flask and shake. Do not shake too vigourously. There will be pressure buildup. Remember to remove the stopper after shaking to prevent pressure buildup.
14) Pour all the solution into the flask and let it sit in the water bath for 10 minutes or until both layers are clearly formed.
15) Careful decant this solution from the flask into the 500mL measuring cylinder. Since the non-polar solvent layer is on top, the majority of it will be poured into the cylinder. NOTE: Do not pour all of the solution in; the cylinder cannot hold it all. Pour as much as the cylinder can hold, giving some leeway to prevent spills.
16) Using a turkey baster, carefully pipette the top non-polar solvent into a rectangular Pyrex dish. Use two hands to keep the turkey baster steady, and pipette by touching the tip of the turkey baster to the side of the cylinder, just below the surface of the non-polar solvent. Stop when you’re getting too close to the basified layer.
17) Using the 1.0mL eyedropper, the remainder of the non-polar solvent can be pipetted. You can rest the palm of your pipetting hand against the side of the cylinder to keep it steady. Your other hand should hold the cylinder to prevent it from falling. The tip of the eyedropper should be resting against the inner surface of the cylinder and just under the surface of the solvent layer.
18 ) Repeat steps 12-17 thrice.
19) Place the rectangular Pyrex dish with the solvent into the freezer. Make sure the lid is locked in. If your dish does not come with a locking lid, then wrap the opening of the dish with cling film and place the entire dish into a large zip lock bag. Zip it up, and place in the freezer. Alternatively, you can let it evaporate completely in a dry place. If evaporating, skip to step 22.
20) Leave the dish in the freezer for a minimum of 24 hours. Do not disturb the dish before 24 hours are up, the crystals may not form properly otherwise.
21) After 24 hours, carefully decant the solvent into another container. It is recommended that you keep the solvent in another glass container for long term storage. This used solvent can be used ad infinitum.
22) Allow the dish to dry, either by focusing a fan into the interior, or letting it evaporate.
23) Scrape up the precipitate with a razor blade, and place on a small section of baking paper. This can be wrapped up, and stored in a cool, dark and dry place.
4. Afterword
So there you have it. You have successfully extracted N,N-Dimethyltryptamine. What you do with this sacred molecule is up to you. “With great power comes great responsibility” - Voltaire.
I would like to give thanks to all the people on the DMT-Nexus who have provided valuable information and support in regards to everything DMT related, and life in general.
A special thanks go out to cyb, chemisTryptaMan and Earthwalker who provided the basic structure of this methodology. All I did was tweak their work. This would not be possible without them. Thanks to some one (that's the username: some one) for pointing out some errors. Also a special shout out to resident chemical supergenius, benzyme.
5. References
cyb, chemisTryptaMan (2013). The cyb/CTM ‘Maximum Yield’ ATB Salt tek MAX ION. Wherever the hell cyb and CTM are from: DMT-Nexus
Earthwalker (2014). ACRB TEK 100g "PICS" (Newbie Friendly). Wherever the hell EW is from: DMT-Nexus
Tro, N. (2011). Chemistry. 2nd ed. Upper Saddle River, New Jersey: Pearson.
Various pictures taken from this textbook and stolen from Google Image searches. Sorry.
2016
Published exclusively on The DMT Nexus.
Published exclusively on The DMT Nexus.
Attachments
-
Principles+of+Alkaloid+Extraction.pdf484 KB · Views: 74
-
Principles+of+Alkaloid+Extraction+2nd+Edition.pdf484.3 KB · Views: 33
-
Principles+of+Alkaloid+Extraction+3rd+Edition.pdf485.5 KB · Views: 21
-
Principles+of+Alkaloid+Extraction+4th+Edition.pdf488.8 KB · Views: 59
-
 ArrheniusBaseCropped.png75.9 KB · Views: 0
ArrheniusBaseCropped.png75.9 KB · Views: 0
Last edited: