Woolmer said:
Thanks Loveall!
Your diagram is labeled slightly differently right?
The way I read it is that the line which is 45 degrees to the NaOH line is the concentration of acetone to water. Upon choosing a concentration, let's say for example 20:80 acetone:water, you can use the tie lines to find the minimal %weight of NaOH (is this the % weight to water or to the entire system?) which must be added to achieve separation which would be around 18%. For my case, I have used 700 ml of water and around 20-30 ml of acetone, so I would likely need around 22% of NaOH which would end up being about 180 grams for an extraction of 70 grams cooperi.
If indeed I am reading this correctly I'd just like to ask whether it would be more beneficial to use much less water as less lye is needed to achieve separation.
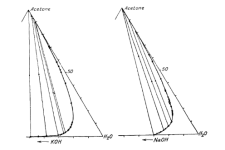
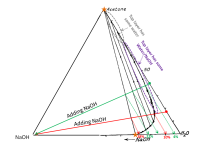
Yes, the video is labeled differently and has different compounds. % are of total weight in the triangle. In your drawing the to top of the triangle is 100% acetone and the bottom right is 100% water. I've added the bottom left which is 100% lye. So your green starting point should be much closer to the water on the bottom right. See image below, I've made your starting green point (before lye) a red point.
As you add lye you move the entire system towards the bottom left of the triangle (which represents 100% lye). To read the amount of lye, draw a line parallel to the right side of the triangle and see where it hits the lye axis (examples are shown with dashed lines)
Let's say for example, you are 80:20 water:acetone by volume. That is ~80:16 by mass. 18% lye for the water means you are now at 80:16:14 by mass. In % this is ~ 73%, 14.5%, 12.5%, it will hardly separate (barely beyond the 10% lye needed to get inside the tie lines). Complete separation would happen at 28%. I've marked these 10% and 28% points in the triangle. This is for a pure system at 0C. Add plant stuff and being at room temperature and things are worse, I would say you need more lye. Regardless, if you keep on adding lye eventually you should get all your acetone (unless some plant oil is messing up the system).
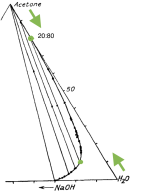
Yes, increasing the acetone/water ratio would be very helpful - especially if this is done by reducing the water. Suppose you start at 40% acetone and 60% water by weight (e.g. you reduced your water before basing and added the same amount of acetone). Then separation starts much sooner (4% total lye), and total separation happens at 22% lye (marked as green in the triangle below).
Notice that the diagram is for 0C. At room temp you will need more lye since lye is more soluble in water at room temp. Conversely, in the cold fridge layers will separate better because less lye is needed to saturate the water. I wonder what happens in the freezer, you may get DMT crystals on top of a frozen water layer (that's just a guess).
Bottom line is simply add more lye until you get almost all or nearly all the acetone back. You don't want to get back a small amount of acetone because those layers could have some NaOH in them (purple region, which I've drawn a bit too large - cuts off sooner towards the bottom because those are really bottom layers). Benz said before to be very basic and high pH for this to work.
The nice thing about this system is how clean the top star is: very pure acetone (as long as enough lye is added). This doesn't always happens (e.g. the system in the earlier video doesn't hit the the pure corner). However, enough lye must be added. The bottom star represents NaOH saturated water. Notice the bottom star is sitting at ~30% NaOH for the total system (with no acetone), that represents 42g of NaOH per 100g of water which is the quoted solubility at 0C
here (since 30% = 42/(100+42).
So at the end of the day, it is very simple. Saturate the water with NaOH (or nearly saturate it) and separation is complete. At room temp this can be a lot of lye - so reducing the water is a good idea.
Also, I would not let this sit for a very long time at warm temps because of the aldol condensation concerns mentioned before (but there may be nothing to this and a simple test showed no issues at room temp for a few hours).
Hope I'm making sense