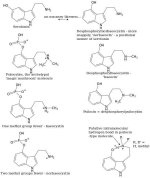
_Trip_ said:It would be interesting to see the whole LSA adducts discussion put to rest.
I bet I could solve that puzzle with charged aerosol detection. Any adduct formed would have a larger droplet size.
It's not like MS, where molecules may fragment at the source. I can run a non-ionic mobile phase, like a pure alcohol (ethanol), and inject intact molecules.