Hehe understandable. SWIM should have everything repaired sometime next week.
-
Members of the previous forum can retrieve their temporary password here, (login and check your PM).
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dehydrobufotenine
- Thread starter 69ron
- Start date
Migrated topic.
men....i just wanted to say that it is truly awesome to read all of your collective chemical genius batting these ideas around. it is because of YOU that we are all able to enjoy these righteous fruits of your labors. just wanted to thank you all from the bottom of my frontal,parietal,temporal and occipital lobes.
LOVE AND GRATITUDE!
LOVE AND GRATITUDE!
Nope got side tracked with other projects  Haven't forgotten though.
Haven't forgotten though.
 Haven't forgotten though.
Haven't forgotten though.FYI: Dehydrobufotenine is a zwitterion. It cannot be freebased.
When SWIM had left his bufotenine to mix in calcium hydroxide for 24 hours, the resulting compound could NOT be freebased. That indicates dehydrobufotenine was made and that Shulgin's theory is right. I would like to see someone test this out to be certain of this reaction.
Dehydrobufotenine has an XLogP3 of 2.3 , while bufotenine has an XLogP3 of 1.2. That means that dehydrobufotenine should cross the blood brain barrier better than bufotenine. Now the results of mixing bufotenine with calcium hydroxide for 24 hours produces a non-smokeable result, but it was never tested sublingually or orally. It’s possible that dehydrobufotenine is active sublingually, orally, or by insufflation. SWIM never tried those routes of ingestion with this substance, he only tried smoking it.
Has anyone else made this and tested its activity other than by smoking it?
When SWIM had left his bufotenine to mix in calcium hydroxide for 24 hours, the resulting compound could NOT be freebased. That indicates dehydrobufotenine was made and that Shulgin's theory is right. I would like to see someone test this out to be certain of this reaction.
Dehydrobufotenine has an XLogP3 of 2.3 , while bufotenine has an XLogP3 of 1.2. That means that dehydrobufotenine should cross the blood brain barrier better than bufotenine. Now the results of mixing bufotenine with calcium hydroxide for 24 hours produces a non-smokeable result, but it was never tested sublingually or orally. It’s possible that dehydrobufotenine is active sublingually, orally, or by insufflation. SWIM never tried those routes of ingestion with this substance, he only tried smoking it.
Has anyone else made this and tested its activity other than by smoking it?
Jorkest said:hmmmm...that is a interesting idea 69ron....SWIM may just have to try out converting some bufo and testing it....would you think that maybe 25mg might be a good starting place for sublingual then to swallow?
That sounds good to me. Whatever the potency is, it’s most likely weaker because snuff left to react for 24 hours is usually said to be weaker not stronger.
By the way, you can safely orally ingest up to 1000 mg of calcium hydroxide per day. Up to 1000 mg of calcium hydroxide is found in some OTC calcium supplements. So don’t worry about traces of food grade calcium hydroxide left in it. You could actually mix 25 mg calcium hydroxide with 25 mg bufotenine in water for 1 day and simply ingest it orally as is without separating it. But if using it sublingually, you’d better separate it because the calcium hydroxide is going to burn like crazy (unlike the stomach, there’s no hydrochloric acid in the mouth to counteract it’s alkalinity).
I bet it’s orally active.
etherealsamba
Rising Star
hey yo there..
sorry to bring up a battered thread..
but on the subject of bufotenine.. as a freebase
it is said that hydroxides will make bufo un-freebaseable..
so a carbonate is suggested..
now, if one were to use calcium carbonate, would any h2o in the process convert this into a hydroxide (calcium hydroxide forms with calcium carbonate and water correct??)
my guess if os, would be to use sodium carbonate instead.. but i am just curious.
thanks .
also, what could oen do to be sure to pull carbonate bufo freebase? would MEK work? what else is bufo indeed soluable in? peace
sorry to bring up a battered thread..
but on the subject of bufotenine.. as a freebase
it is said that hydroxides will make bufo un-freebaseable..
so a carbonate is suggested..
now, if one were to use calcium carbonate, would any h2o in the process convert this into a hydroxide (calcium hydroxide forms with calcium carbonate and water correct??)
my guess if os, would be to use sodium carbonate instead.. but i am just curious.
thanks .
also, what could oen do to be sure to pull carbonate bufo freebase? would MEK work? what else is bufo indeed soluable in? peace
hey, bufo freebase is soluble in ipa and acetone, check this threadetherealsamba said:hey yo there..
sorry to bring up a battered thread..
but on the subject of bufotenine.. as a freebase
it is said that hydroxides will make bufo un-freebaseable..
so a carbonate is suggested..
now, if one were to use calcium carbonate, would any h2o in the process convert this into a hydroxide (calcium hydroxide forms with calcium carbonate and water correct??)
my guess if os, would be to use sodium carbonate instead.. but i am just curious.
thanks .
also, what could oen do to be sure to pull carbonate bufo freebase? would MEK work? what else is bufo indeed soluable in? peace
PsilocybeChild
Rising Star
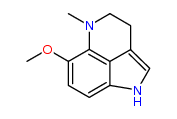
Dehydrobufotenine is a phenolic quaternary amine indole. Unlike bufotenine, dehydrobufotenine is not truly a tryptamine. It lacks the typical tryptamine side chain. However, both bufotenine and dehydrobufotenine are indoles. Dehydrobufotenine contains the same 5-Hydroxyindole structure contained in bufotenine. Instead of the tryptamine side chain as bufotenine has, dehydrobufotenine contains a piperidine structure similar to 1,1-dimethylpiperidinium. This gives dehydrobufotenine some structural similarity to the indole side of LSD's chemical structure.
Dehydrobufotenine has 1 less hydrogen atom than bufotenine. It can be prepared by dehydrogenation of bufotenine.
Hydrogenation of dehydrobufotenine yields bufotenine.[5] Bufotenine can be produced from dehydrobufotenine by an Emde-type fission.[4]
Human Metabolism
The human metabolism of dehydrobufotenine is currently unknown. It's metabolism appears not to have been studied in either humans or animals.
THE REST OF THIS SECTION IS PURE CONJECTURE
Quaternary amines are typically not substrates of Monoamine Oxidase A or Monoamine Oxidase B. Bufotenine, which dehydrobufotenine is derived from, and the related psilocin are minor substrates of Monoamine Oxidase A. Dehydrobufotenine is likely not affected by either enzyme.
The related psilocin is a major substrate of Glucuronosyltransferases UGT1A9 and UGT1A10. Bufotenine is also a major substrate of Glucuronosyltransferase in rats, and is likely to also be so in humans. Dehydrobufotenine is potentially also a substrate of Glucuronosyltransferase. Because it should not be a substrate of either Monoamine Oxidase A or Monoamine Oxidase B, Glucuronosyltransferase is likely to be a major enzyme involved in dehydrobufotenine metabolism. If correct, then the major metabolite of dehydrobufotenine should be dehydrobufotenine glucuronide.
Psychoactivity
The psychoactivity of dehydrobufotenine remains unstudied in humans. Anonymous reports give the low dose range as 2-10 mg orally, producing stimulation, euphoria, and mild psychoactive effects. Effects begin within about 30 minutes after ingestion. Peak after about 3 hours. Total effects last approximately 12-16 hours. Larger doses are reported to have full blown psychedelic effects similar to LSD and mescaline, and unlike those of bufotenine or psilocin. The long duration of effects is likely attributed to it being a quaternary amine. Quaternary amines are typically not substrates of Monoamine Oxidase A or Monoamine Oxidase B.
Attachments
downwardsfromzero
Boundary condition
It strikes me that a methyl group might easily transfer from the quaternary nitrogen to the hydroxy group very close nearby via a five-membered cyclic transition state. This would yield O-methylnordehydrobufotenine -the cyclised equivalent of 5-MeO-NMT, which Shulgin mentioned somewhere. That's a molecule that looks like it would be active, too.
entheogenic-gnosis
Rising Star
downwardsfromzero said:It strikes me that a methyl group might easily transfer from the quaternary nitrogen to the hydroxy group very close nearby via a five-membered cyclic transition state. This would yield O-methylnordehydrobufotenine -the cyclised equivalent of 5-MeO-NMT, which Shulgin mentioned somewhere. That's a molecule that looks like it would be active, too.
O-Methylnordehydrobufotenine: This is a rearrangement product of dehydrobuftenine, which may be a natural product or it may be an artifact of analysis.
-shulgin;TIHKALErowid Online Books : "TIHKAL" - #19 5-HO-DMT
Entry #19 5-HO-DMT from TiHKAL by Alexander & Ann Shulgin.erowid.org
This passage appears in my printed copy of TIHKAL, however erowid's online version of TIHKAL left this last paragraph out, I'm not sure why, it was probably a mistake:
A fascinating cyclization product of this "nor-compound" [5-meo-NMT] is a cyclic dehydrogenation product where there is a direct coupling of the tryptamine nitrogen to the 4 position of the indole ring. This tricyclic material, O-methyl-nor-dehydrobufotenine proved to be of comparable activity to DMT in rat studies, but has not apparently been studied in man -shulgin;TIHKAL
More related information concerning potential activity of O-methylnordehydrobufotenine:
I am on a tablet and can not copy and paste from PDF files, this was the only available section I could find which I was able to post here, however I highly recommend downloading and reviewing the entire article via PDF file which can be accessed in the link below:
In this test, the O-methylnordehydrobufotenine is approximately twice as active as mescaline (ED50 = 71.0 umoles/kg), but is much less active than its open-chain analog, N,N-dimethyl-5-methoxytryptamine, which from published data can be estimated to be much more than 30 times as active a hallucinogen as mescaline. When injected subcutaneously into NIH general purpose white mice, O-methylnordehydrobufotenine at 20 mg/kg causes only slight overt changes (reduction in spontaneous activity) while N,N-dimethyl-5-methoxy-tryptamine at 10 mg/kg causes profound effects. At this dosage the mice lose the ability to move normally and engage in locomotor activity with legs extended laterally.
-The Synthesis of O-Methylnordehydrobufotenine, a New Psychoactive Indole.
Fred G. H. Lee, John W. Daly, and Albert A. Manian
J. Med. Chem. 12 (1969) 321-322
We discussed something very similar in THIS thread sometime back, so I'm sorry if this is too similar to post 9 of that thread, but it is relevant here well.
Structurally O-methylnordehydrobufotenine is quite similar to RU-28306 or Bay R 1531 or even LY-293284. RU-28306 being the most interesting in my mind. When I was younger, and had first noticed the mono-methyl-tryptamine moiety burried within the LSD molecule, I figured that if you could add a second methyl group to nitrogen 6 of LSD that you would in turn produce a molecule which would essentially be LSD with a dimethyltryptamine moiety burried within it, but the nitrogen at position 6 of LSD does not have any available valence electrons to facilitate adding this additional nitrogen, so the idea died there, however, RU-28306 could be seen as an LSD/DMT moiety combination, only an entire ring structure had to be basically removed.
RU-28306 is a tricyclic tryptamine derivative which acts as a serotonin receptor agonist, with selectivity for 5-HT1 and 5-HT2 subtypes. It can be regarded either as a conformationally constrained derivative of DMT, or a structurally simplified analogue of LSD, but the binding affinity of racemic RU-28306 is closer to that of DMT, though with relatively higher affinity for 5-HT2 subtypes and lower for 5-HT1. -Wikipedia
There really is not much useful information regarding RU-28306, when I searched all I found was some individuals on sites which I do not use, who seemed to be seeking to get intoxicated, who asking about it, completely useless. As far as research information, not much is out there, I think I remember having had an article or two, but can not locate them.
Brief info on the other tricyclic tryptamine which is structurally similar to O-methylnordehydrobufotenine, bay R 1531.
Bay R 1531 is a tricyclic tryptamine derivative which acts as a selective serotonin receptor 5-HT1A agonist. It was researched unsuccessfully for the treatment of stroke but remains in use for scientific research -Wikipedia
It's been a while since I have done research regarding these molecules...I have actually learned a ton since the last time I had posted regarding these compounds, the funny thing was, school was only good for the basics, and I was not learning very efficiently, I also could not mention anything relating to psychedelic or psychoactive chemistry in school or to my professors for obvious reasons. It was not until I started working with other chemists outside of school that I really began to learn, I recently took on an apprenticeship with a retired organic chemistry professor, who has a fairly nice laboratory. It was through this recent one on one and hands on learning that I really began to excel and grow at the art of organic chemistry. In the last year and a half I have made more progress than I did with 3 years in school. Though it's all beneficial in the end, every day I learn something new and progress a little further.
Any way, this is getting lengthy and I'm straying off topic periodically, so I will stop here.
Pictures attached are O-methylnordehydrobufotenine, RU-28306 and Bay R 1531.
-eg
Attachments
downwardsfromzero
Boundary condition
Thanks. That's exactly what I was thinking of, although perhaps a link to the other thread would have sufficed for the latter part 
That BAYR 1531 is also a dipropylaminotetralin (DPAT) derivative. DPAT derivatives also have some interesting properties. Some fall into the class known as 'serenics', for example.

That BAYR 1531 is also a dipropylaminotetralin (DPAT) derivative. DPAT derivatives also have some interesting properties. Some fall into the class known as 'serenics', for example.
entheogenic-gnosis
Rising Star
I'm sure a good deal of these 5HT1 agonists will prove to be "serenic" in nature, as long as its an "agonist that mainly acts on the postsynaptic 5-HT(1A/1B) receptor sites"...
I'm still quite interested in O-Methylnordehydrobufotenine, as well as RU-28306.
Just curious, Are there any 12-methoxy substituted LSD homologues? Or 12 substituted lysergamide compounds in general?
-eg
The well-known fact that certain 5-HT(1A/1B) receptor agonists potently and specifically reduce aggressive behavior without motor slowing and sedative effects is only consistent with this hypothesis under the assumption that the agonist mainly acts on the postsynaptic 5-HT(1A/1B) receptor sites.

5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis - PubMed
More than any other brain neurotransmitter system, the indolamine serotonin (5-HT) has been linked to aggression in a wide and diverse range of species, including humans. The nature of this linkage, however, is not simple and it has proven difficult to unravel the precise role of this amine in...www.ncbi.nlm.nih.gov
I'm still quite interested in O-Methylnordehydrobufotenine, as well as RU-28306.
Just curious, Are there any 12-methoxy substituted LSD homologues? Or 12 substituted lysergamide compounds in general?
-eg
entheogenic-gnosis
Rising Star
downwardsfromzero said:Thanks. That's exactly what I was thinking of, although perhaps a link to the other thread would have sufficed for the latter part
That BAYR 1531 is also a dipropylaminotetralin (DPAT) derivative. DPAT derivatives also have some interesting properties. Some fall into the class known as 'serenics', for example.
If you feel the later part is not necessary, I would gladly remove it to improve the thread, just let me know.
-eg
downwardsfromzero
Boundary condition
I've often wondered this myself. Kind of the LSD equivalent of 5-MeO-DMT. That's an area of my literature research that really ought to move forward.Just curious, Are there any 12-methoxy substituted LSD homologues? Or 12 substituted lysergamide compounds in general?
To some extent this ties in as well with David Nichols' work where it was found that LSD is metabolised by certain people to form 13-hydroxy-LSD which is a potent dopaminergic agonist and has been linked to the 'dark' aspect that possibly these same certain people experience in the latter part of an LSD trip? Somewhere there's a video where Nichols covers this.
entheogenic-gnosis
Rising Star
downwardsfromzero said:I've often wondered this myself. Kind of the LSD equivalent of 5-MeO-DMT. That's an area of my literature research that really ought to move forward.Just curious, Are there any 12-methoxy substituted LSD homologues? Or 12 substituted lysergamide compounds in general?
To some extent this ties in as well with David Nichols' work where it was found that LSD is metabolised by certain people to form 13-hydroxy-LSD which is a potent dopaminergic agonist and has been linked to the 'dark' aspect that possibly these same certain people experience in the latter part of an LSD trip? Somewhere there's a video where Nichols covers this.
Interesting. Thanks.
I'm actually surprised more has not been done regarding this matter.
I've noticed the last few hours of an LSD experience can be almost amphetamine like, LSD itself does affect dopamine receptor subtypes, as well as adrenoreceptor subtypes, and I've always assumed that this was the source of the stimulant effect encountered in the later LSD experience. I had not considered metabolites, and since this effect is occurs in the later end of the experience this would have been logical.
I'll search out the Nichols video.
-eg
downwardsfromzero
Boundary condition
entheogenic-gnosis
Rising Star
downwardsfromzero said:
Awesome, thanks.
I always take Sundays off the nexus, so Monday there's a good deal to read and write, I would have gotten to this faster otherwise, again, thanks.
-eg
Similar threads
- Replies
- 7
- Views
- 1K
- Replies
- 10
- Views
- 1K