-
Members of the previous forum can retrieve their temporary password here, (login and check your PM).
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
TEK Ethyl acetate approach [CIELO]
- Thread starter Loveall
- Start date
This topic contain a TEK
Migrated topic.
Cheelin said:One approach that may help all who find a second liquid layer forming during salting is to heat up the solvent (no sparks or open flame) and stir to dissolve the goo back into solution, then let cool. The problem is that crystal nucleation was disrupted somehow, and further crystallization stalled; so try to restart the process, and add additional steps, as necessary.
For what it's worth in my recent goo case I did try heating up my jar ~140F. (jar in container of water that was <150F at start) It was well after initially salting though after I added additional CA when the goo seemed to thicken up some more... so probably too late at that point. I probably should've mentioned I tried that at the time, as well as putting it on mag stirrer for awhile after the addtl. CA seemed to thicken it up.
starbob said:For what it's worth in my recent goo case I did try heating up my jar ~140F. (jar in container of water that was <150F at start) It was well after initially salting though after I added additional CA when the goo seemed to thicken up some more... so probably too late at that point. I probably should've mentioned I tried that at the time, as well as putting it on mag stirrer for awhile after the addtl. CA seemed to thicken it up.
That's consistent with what I've tried too. I did not see any appreciable increase in solubility of the oil all the way up to the boiling point of EA.
It does look like the oil is a mixture of CA, mescaline citrate and probably water, maybe other contaminants, based on the acidity of fresh solvent that I allowed to stand over the solidified oil for over a week while crystals formed. Even after all this time some has still not converted. I plan to try Nemoo's advice to try trituration under acetone with some oiled out jars I still have hanging around.
With regards to using K2CO3 for drying, the one trial I did suggests there are drawbacks. While it enabled me to crystallize the citrate using the passive method, the vast majority of the crystals were stuck to the walls of the jar. The fumarate precipitated rapidly as a fine sparkly powder instead of crystals. I ended up using vacuum filtration with a very fine filter paper to ensure I collected it all. I opted to recrystallize both (separately of course) from water to consolidate them into something easier to collect.
From what I've observed so far, fumarate is definitely more resistant to excess water, but probably still benefits from the incomplete removal of water that sodium carbonate provides.
Thinking about crystallization, the general idea of heating solvent and dissolving 2nd phase, the dissolving should be done as early as possible. Also, the research describes general case of heating solvent to supersaturation, then cooling to crystallize. In this case, we are using an acid to the job of the temperature change to aggregate the M for nucleation. So, perhaps the acid needs to be neutralized before trying to dissolve the second phase. This could perhaps be accomplished by decanting the primary phase (EA) and replacing with fresh EA before heating/dissolving, OR by chemically neutralizing the pH of the existing EA prior to heating/dissolving.starbob said:Cheelin said:One approach that may help all who find a second liquid layer forming during salting is to heat up the solvent (no sparks or open flame) and stir to dissolve the goo back into solution, then let cool. The problem is that crystal nucleation was disrupted somehow, and further crystallization stalled; so try to restart the process, and add additional steps, as necessary.
For what it's worth in my recent goo case I did try heating up my jar ~140F. (jar in container of water that was <150F at start) It was well after initially salting though after I added additional CA when the goo seemed to thicken up some more... so probably too late at that point. I probably should've mentioned I tried that at the time, as well as putting it on mag stirrer for awhile after the addtl. CA seemed to thicken it up.
Again, the issue here is disrupted nucleation, understand the mechanics of crystallization, then develop possible fixes based on that knowledge.
Cheelin said:Thinking about crystallization, the general idea of heating solvent and dissolving 2nd phase, the dissolving should be done as early as possible. Also, the research describes general case of heating solvent to supersaturation, then cooling to crystallize. In this case, we are using an acid to the job of the temperature change to aggregate the M for nucleation. So, perhaps the acid needs to be neutralized before trying to dissolve the second phase. This could perhaps be accomplished by decanting the primary phase (EA) and replacing with fresh EA before heating/dissolving, OR by chemically neutralizing the pH of the existing EA prior to heating/dissolving.starbob said:Cheelin said:One approach that may help all who find a second liquid layer forming during salting is to heat up the solvent (no sparks or open flame) and stir to dissolve the goo back into solution, then let cool. The problem is that crystal nucleation was disrupted somehow, and further crystallization stalled; so try to restart the process, and add additional steps, as necessary.
For what it's worth in my recent goo case I did try heating up my jar ~140F. (jar in container of water that was <150F at start) It was well after initially salting though after I added additional CA when the goo seemed to thicken up some more... so probably too late at that point. I probably should've mentioned I tried that at the time, as well as putting it on mag stirrer for awhile after the addtl. CA seemed to thicken it up.
Again, the issue here is disrupted nucleation, understand the mechanics of crystallization, then develop possible fixes based on that knowledge.
If after neutralizing it doesn't crystallize, the process may need to be backed up further, may have to actually re-basify after heating/dissolving, then slowly titrate the acid when re-salting while stirring.
Cheelin said:If after neutralizing it doesn't crystallize, the process may need to be backed up further, may have to actually re-basify after heating/dissolving, then slowly titrate the acid when re-salting while stirring.Cheelin said:Thinking about crystallization, the general idea of heating solvent and dissolving 2nd phase, the dissolving should be done as early as possible. Also, the research describes general case of heating solvent to supersaturation, then cooling to crystallize. In this case, we are using an acid to the job of the temperature change to aggregate the M for nucleation. So, perhaps the acid needs to be neutralized before trying to dissolve the second phase. This could perhaps be accomplished by decanting the primary phase (EA) and replacing with fresh EA before heating/dissolving, OR by chemically neutralizing the pH of the existing EA prior to heating/dissolving.starbob said:Cheelin said:One approach that may help all who find a second liquid layer forming during salting is to heat up the solvent (no sparks or open flame) and stir to dissolve the goo back into solution, then let cool. The problem is that crystal nucleation was disrupted somehow, and further crystallization stalled; so try to restart the process, and add additional steps, as necessary.
For what it's worth in my recent goo case I did try heating up my jar ~140F. (jar in container of water that was <150F at start) It was well after initially salting though after I added additional CA when the goo seemed to thicken up some more... so probably too late at that point. I probably should've mentioned I tried that at the time, as well as putting it on mag stirrer for awhile after the addtl. CA seemed to thicken it up.
Again, the issue here is disrupted nucleation, understand the mechanics of crystallization, then develop possible fixes based on that knowledge.
I’d be interested in seeing orchidist do a stirring-acid titration run with his goo powder. Perhaps taking pH measurements of fresh EA, combined pulls, and post-salting solution.
downwardsfromzero
Boundary condition
- Merits
- 3
I hope to be able to add some more information about the butyl acetate variation at some point, for those of us who find that solvent easier (and cheaper) to get hold of than EA. I've seen that BA can in principle work with harmalas but its utility with cactus is not entirely clear. This is because initial tests were only carried out with cactus of questionable quality. The small amount of resulting crystal looked wrong and its identity has not been confirmed.
What we do know from the harmala tests is that it's necessary to add water to the BA in order to get the citric acid to dissolve properly. A solution of citric in damp BA can then be added to the alkaloidal extract - whether this moisture will result in goo if using cactus remains to be seen.
What we do know from the harmala tests is that it's necessary to add water to the BA in order to get the citric acid to dissolve properly. A solution of citric in damp BA can then be added to the alkaloidal extract - whether this moisture will result in goo if using cactus remains to be seen.
downwardsfromzero said:Meanwhile... I hope to be able to add some more information about the butyl acetate variation at some point, for those of us who find that solvent easier (and cheaper) to get hold of than EA. I've seen that BA can in principle work with harmalas but its utility with cactus is not entirely clear. This is because initial tests were only carried out with cactus of questionable quality. The small amount of resulting crystal looked wrong and its identity has not been confirmed.
What we do know from the harmala tests is that it's necessary to add water to the BA in order to get the citric acid to dissolve properly. A solution of citric in damp BA can then be added to the alkaloidal extract - whether this moisture will result in goo if using cactus remains to be seen.
Interesting stuff! It would be exciting if this TEK does extend to work with BA. Perhaps at some point I can get a hold of some myself and we can try for replication if you end up with a good result, especially since I've apparently got the gooperpowers when it comes to citric acid :lol:.
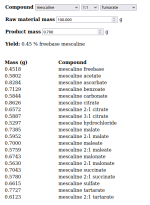
I posted about this in the salts to freebase calculator thread earlier, wanted to cross post it here because I think it'll be useful to many who try the TEK, and since it's browser based, doesn't require any spreadsheet software.
I've just added the ability to automatically produce yield % (standardized on the freebase to maintain equivalence between TEKS using different acids) and added some documentation.
Link to the repo is here
Planning to add an interactive CIELO ingredient calculator too.
I've just added the ability to automatically produce yield % (standardized on the freebase to maintain equivalence between TEKS using different acids) and added some documentation.
Link to the repo is here
Planning to add an interactive CIELO ingredient calculator too.
Attachments
Just a quick update, I'm going to work on optimizing my paste ratio/yield for a few runs and try to find the tipping point for oil with citric acid.
For these runs, I'll be leaving out any drying agents, and doing two runs a day using the same paste ratio, one salted with fumaric acid, the other citric acid.
Today though, I'm also doing a third run with succinic acid to satisfy the mycotopia people. If anyone could vouch for me to get out of probation there, I've registered with the same name
For these runs, I'll be leaving out any drying agents, and doing two runs a day using the same paste ratio, one salted with fumaric acid, the other citric acid.
Today though, I'm also doing a third run with succinic acid to satisfy the mycotopia people. If anyone could vouch for me to get out of probation there, I've registered with the same name

- Merits
- 42
the 'goo' or the crashing out of a liquid instead of a crystal is commonly seen in a crystallization gone wrong. It can happen with neutral molecules, as well as salts, with similar but differing properties that can lead to this kind of solute-to-liquid phase transition. Sometimes, the crystallization can occur immediately after the the solute-to-liquid transition, this is usually desirable if it happens fast enough, but if it doesn't happen right away, its usually a disaster and the product will not be as pure as desired and not clean looking either, and usually will require a second recrystallization. This has happened to me most frequently with higher n-alkyl tryptamines (especially N-alkyl-N,isopropyl) and their salts. This is also the original reason why chemists working with DMT stopped using the standard hydrochloride salt, because DMT HCl was more prone to goo-formation (better to think of it like an ionic liquid) than it was to crystal formation.
The actual reasons for these amphorous goos or liquid salts is complicated and can vary depending on the counterion. Citrate is another one of these salts that classically can be more prone to amorphous solids or goo, but it shouldn't be assumed that it is like this in all cases, quite the contrary, it is possible to learn to control the conditions of a particular process, and end up with a crystalline product every time.
In my experience, when I was working with a particular molecule that was giving me this problem, I would often start by making sure the solvent had been well dried, if this did not fix the issue by itself. Then I had found that adding the acid too fast, or adding excess acid, especially in a concentrated solution, would often be the culprit. Citric acid is a triprotic acid with an alcohol group, so once its in its liquid/goo form you can expect a lot of intermolecular hydrogen bonding, but I'm not too confident in theorizing what particular interaction is going to promote liquid to liquid versus liquid to solid here. What I do know, if you keep a tight control on the concentration, and a tight control on the rate of addition from acid, and a little patience, you usually get a successful formation of crystals. Basically, concentration, temperature, and the rate of neutralization are the 3 single most important factors, after solvent and solute (and counterion). So start with adding a bit more solvent to dilute, then add the neutralizing acid (citric) dropwise, stirring with each addition. Some people like to use a magnetic stirrer for this, I prefer to do it by hand, add a few drops, swirl the container, and observe crystal formation. Ideally, you should wait to add anymore acid until you have observed some nucleation. Then you are good to go. Then you continue carefully, until you reach the end point. Because in some cases excess acid can cause all the nice crystals to turn to goo, I will use pH papers to roughly test when I am nearing the end point. I highly recommend first dissolving the citric acid in some solvent, either in ethyl acetate or in acetone or isopropanol, neither will affect the solubility of the final product, and will greatly aid in your control of the dropwise addition of acid, and the visual process of crystallization. Again, if you have found a process that works for you, and you are happy with the product, then keep doing it, there isn't one way to do anything.
The actual reasons for these amphorous goos or liquid salts is complicated and can vary depending on the counterion. Citrate is another one of these salts that classically can be more prone to amorphous solids or goo, but it shouldn't be assumed that it is like this in all cases, quite the contrary, it is possible to learn to control the conditions of a particular process, and end up with a crystalline product every time.
In my experience, when I was working with a particular molecule that was giving me this problem, I would often start by making sure the solvent had been well dried, if this did not fix the issue by itself. Then I had found that adding the acid too fast, or adding excess acid, especially in a concentrated solution, would often be the culprit. Citric acid is a triprotic acid with an alcohol group, so once its in its liquid/goo form you can expect a lot of intermolecular hydrogen bonding, but I'm not too confident in theorizing what particular interaction is going to promote liquid to liquid versus liquid to solid here. What I do know, if you keep a tight control on the concentration, and a tight control on the rate of addition from acid, and a little patience, you usually get a successful formation of crystals. Basically, concentration, temperature, and the rate of neutralization are the 3 single most important factors, after solvent and solute (and counterion). So start with adding a bit more solvent to dilute, then add the neutralizing acid (citric) dropwise, stirring with each addition. Some people like to use a magnetic stirrer for this, I prefer to do it by hand, add a few drops, swirl the container, and observe crystal formation. Ideally, you should wait to add anymore acid until you have observed some nucleation. Then you are good to go. Then you continue carefully, until you reach the end point. Because in some cases excess acid can cause all the nice crystals to turn to goo, I will use pH papers to roughly test when I am nearing the end point. I highly recommend first dissolving the citric acid in some solvent, either in ethyl acetate or in acetone or isopropanol, neither will affect the solubility of the final product, and will greatly aid in your control of the dropwise addition of acid, and the visual process of crystallization. Again, if you have found a process that works for you, and you are happy with the product, then keep doing it, there isn't one way to do anything.
Thanks Mindlusion, that pretty much all agrees with what I'm seeing in my trials. I'm extremely happy with the results I get from fumaric acid, but I do want to continue on with the challenge of making citrate crystals reliably. Drying definitely helped, although in all cases the fumarate formed crystals far faster. Often within 10 minutes. Salting with a more controlled addition of CA in solvent as you described is on my to-do list.
Added succinic acid to a jar about ten minutes ago. Initially, clouding was slow, but now the jar is uniformly cloudy. I'm using pretty conservative, though still in excess of my expected yield, quantities of acid for these runs. In this case, 137mg on a 20g extraction, a bit more than 200mL of solvent, but most of the acid has already dissolved.
No crystals yet, but if it crystallizes reliably and has better solubility compared to fumaric acid, it might to replace fumaric acid as my favorite.
No crystals yet, but if it crystallizes reliably and has better solubility compared to fumaric acid, it might to replace fumaric acid as my favorite.
Congrats on all your recent success! That succinate jar is beautiful!orchidist said:Woke up to find all three jars, fumarate, citrate and succinate had crystallized.
The succinate was especially pretty, a single mass of super fine needles at the bottom...I really like how that turned out
- Merits
- 96
orchidist said:Woke up to find all three jars, fumarate, citrate and succinate had crystallized.
The succinate was especially pretty, a single mass of super fine needles at the bottom...I really like how that turned out!
Congrats. So what do you think helped you with the citrate goo you were getting originally? Do you still think fumaric or succinic is more robust in your case?
Congrats on all your recent success! That succinate jar is beautiful!
It was almost too pretty to decant
 ! And it held together well enough when I moved it, that it might've survived as a decoration if I wanted to!
! And it held together well enough when I moved it, that it might've survived as a decoration if I wanted to! Loveall said:Congrats. So what do you think helped you with the citrate goo you were getting originally? Do you still think fumaric or succinic is more robust in your case?
I reduced the water content in the paste, and that seems to have worked. For 20.05g cactus, I used 55.38g of water (92.3% of the maximum recommended amount). I reproduced this paste three times. Consistency looked good. I'll up the percentage of water in my next set of runs. A confounding variable could be that my workspace got progressively colder as the night went on. Started at 58F, by the time I got to the succinate pulls, the room was 45F.
I'll be reporting all my yield percentages converted to freebase for consistency.
For reference, my previous run using the full 3:1 ratio, salted with fumaric acid had a yield of 0.45%.
Citrate weighed in at 137mg, which is 0.36% freebase
Fumarate weighed in at 101mg, which is 0.33% freebase
Succinate weighed in at 80mg, which is 0.26% freebase
The succinate yield goes up to 0.31% if I calculate it as the dimescaline salt. I am inclined to think that it accounts for the lower yield, but I want more data points before I feel certain.
I checked the individual pulls for clouding by placing a drop of the pull on a glass plate with a crystal of citric acid, and holding it up to light. I did not observe any clouding by the end.
I'm attaching pictures of my containers where I'm pooling my fumarate product and the new one with the succinate. The succinate is beautifully white and cottony in consistency, so I think it'll be really easy pack into capsules, hopefully I can tune it to get the yields up and make it my new favorite.
Attachments
- Merits
- 96
orchidist said:Citrate yields have been updated here
Thanks.
So less water in the paste helped with goo in your case? That's interesting.
Another thing to note is that your cactus is yielding on the lower side (0.3%).
Perhaps lower yielding cacti xtalization is more sensitive to citrate. While Cheelin and I both xtalized additional pulls 6-10 with very low yields, by then other plant matter pulled was low also (we were checking for extraction completenes in pulls 1-5.
If anyone gets citrate goo, them gets xtals with fumaric, succinic, or other anions, please report it here with yields. Wondering if a consistent theme can emerge here.
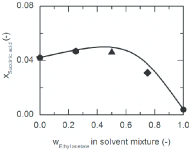
PS: I believe succinic acid solubility in EA is ~25mg/g based on the plot below (eyeballing X=0.002 in pure EA)
Attachments
Similar threads
- Replies
- 8
- Views
- 582
- Replies
- 10
- Views
- 926
- Replies
- 8
- Views
- 2K