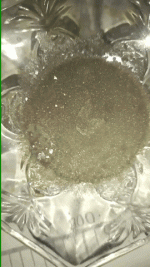
Brennendes Wasser said:So I wrote in regards of Woolmer that the tan colour seems just like what I had observed for Bufo Benzoate. But then I again read your wiki article that shows super nice clear crystals of Bufo Benzoate. That would then make up the question why seems in our case Bufo Benzoate be more of a dirty tan opaque powder instead of your crystal clear beauty crystals? Actually expecting a pure product the nice pictures in your TEK look much more of a reasonable candidate for a pure product. And therefore I believe what you actually have received might be the true Bufo Benzoate instead of the dirty stuff that we have obtained.
The actual difference is:
I took some Bufo Freebase and converted it to Bufo Benzoate in the minimal amount of liquids to maintain minimal solubility. Woolmer took EA and then evaporated it further down. That means both our products were crystallized at pretty harsh conditions, while you crystallized your Bufo Benzoate straight from the extraction liquid. Therefore I am sure your conditions were much smoother, potentially resulting in a slower and therefore well-ordered crystallization.
BW please see the method I used in post #69 and the final results in post #71.
I never used EA or evaporated anything. It was acetone pulls without any prior defatting to which benzoic acid was added. After each addition of BA, the colour got whiter as is seen in post #71. I am sure a prior defat would have yielded a whiter product.
A thought I have re _Trip_'s larger crystals is that he left the acetone pulls for a few days (post #68 ) after which it would have picked up some water thereby increasing the solubility of bufo benzoate and leading to slower crystallization. Was your acetone dry before the extraction?
My acetone was dried beforehand and kept as dry as possible during extraction. After the pulls, it was dried again. The addition of BA immediately made small chunks of precipitate and after 20 minutes a sheet would cover the bottom of the dish.
I think adding water or not drying your acetone could lead to larger crystals, but may decrease yield. Should not be difficult to test I may give this a go.
Brennendes _Trip_ said:Would love to hear more feedback on activeness of the crystals when you're up to explore it further Woolmer. I think lab testing would really bring this home and give us a good idea of what's exactly being yielded.
I will have more chance to assay in a few week's time. Unfortunately, I think it will be very long before lab test results come in. Our postal system is very poorly run and packages take months to reach their destination, if they ever do. I will try to get hold of some TLC plates in the meantime to do qualitative analysis.